Olaparib-Cediranib作为PARP-VEGFR3双抑制剂的抗肿瘤效果:电荷转换pH响应纳米制剂的合理设计、合成、生物学评价和构建
IF 4.7
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
受多聚腺苷核糖聚合酶(PARP)和血管内皮生长因子受体(VEGFR)抑制剂联合使用的临床效果的影响,基于Olaparib (PARP抑制剂)和Cediranib (VEGFR抑制剂)的结构共性,提供了双PARP-VEGFR抑制组合的纲要。上述努力最终形成了一种非常有效的抗肿瘤剂18,具有显著的细胞生长抑制作用[IC50 = 0.37 μM (HL60);0.56 μm (a549);0.80 μM (MDA-MB-231)]通过平衡抑制PARP和VEGFR。具体来说,其中18个表现出出色的PARP1、PARP2和VEGFR3抑制谱,IC50值分别为0.0763 nM、0.0366 nM和4.25 nM。此外,开发了一种电荷可转换的聚合物纳米制剂(18- peg - pll /DMMA纳米颗粒),用于靶向药物递送18。令人鼓舞的是,由于其ph敏感行为,纳米制剂对正常细胞表现出细胞毒性,并表现出对实体瘤细胞的选择性细胞生长抑制,可能是由于EPR效应。本文章由计算机程序翻译,如有差异,请以英文原文为准。
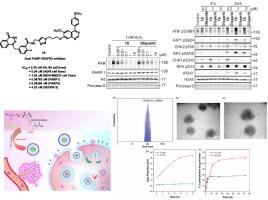
Olaparib-Cediranib Hybrid as Dual PARP-VEGFR3 Inhibitor Elicits Antitumor Efficacy: Rational Design, Synthesis, Biological Evaluation and Construction of a Charge Convertible pH Responsive Nanoformulation
Tempted by the clinical outcomes attained with the combination of poly(ADP-ribose) polymerase (PARP) and vascular endothelial growth factor receptor (VEGFR) inhibitors, a compendium of dual PARP-VEGFR inhibitory assemblages based on structural commonalities of Olaparib (PARP inhibitor) and Cediranib (VEGFR inhibitor) was furnished. The aforementioned efforts culminated into a strikingly potent antitumor agent 18 exerting remarkable cell growth inhibitory effects [IC50 = 0.37 μM (HL60); 0.56 μM (A549); 0.80 μM (MDA-MB-231)] through a balanced inhibition of PARP and VEGFR. Specifically, 18 displayed magnificent PARP1, PARP2, and VEGFR3 inhibitory profiles with an IC50 value of 0.0763 nM, 0.0366 nM, and 4.25 nM, respectively. Further, a charge-convertible polymer-based nanoformulation (18-PEG-PLL/DMMA nanoparticles) for targeted drug delivery of 18 was developed. Encouragingly, the nanoformulation demonstrated a cytotoxicity-devoid profile towards normal cells owing to its pH-sensitive behavior and manifested selective cell growth inhibition against solid tumor cells, possibly due to the EPR effect.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioorganic Chemistry
生物-生化与分子生物学
CiteScore
9.70
自引率
3.90%
发文量
679
审稿时长
31 days
期刊介绍:
Bioorganic Chemistry publishes research that addresses biological questions at the molecular level, using organic chemistry and principles of physical organic chemistry. The scope of the journal covers a range of topics at the organic chemistry-biology interface, including: enzyme catalysis, biotransformation and enzyme inhibition; nucleic acids chemistry; medicinal chemistry; natural product chemistry, natural product synthesis and natural product biosynthesis; antimicrobial agents; lipid and peptide chemistry; biophysical chemistry; biological probes; bio-orthogonal chemistry and biomimetic chemistry.
For manuscripts dealing with synthetic bioactive compounds, the Journal requires that the molecular target of the compounds described must be known, and must be demonstrated experimentally in the manuscript. For studies involving natural products, if the molecular target is unknown, some data beyond simple cell-based toxicity studies to provide insight into the mechanism of action is required. Studies supported by molecular docking are welcome, but must be supported by experimental data. The Journal does not consider manuscripts that are purely theoretical or computational in nature.
The Journal publishes regular articles, short communications and reviews. Reviews are normally invited by Editors or Editorial Board members. Authors of unsolicited reviews should first contact an Editor or Editorial Board member to determine whether the proposed article is within the scope of the Journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


