盐水-油界面在理解沥青质的界面行为中的相关性
IF 5.2
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
Langmuir压力-面积(π-A)等温线被用来研究沥青质界面膜的行为,特别是在简单的水-空气界面。然而,为了理解它们在复杂界面中的行为,比如原油乳剂中的行为,更真实的界面是必不可少的。本文通过SEM显微镜、EDS、CHN元素分析和FTIR对沥青质进行了表征,并利用π-A等温线和流变学测量研究了沥青质膜在水-空气、水-油和盐水-油界面上的行为。结果表明,界面性质和沥青质浓度决定了膜的刚性和分子组织。在水-空气中,增加沥青质的初始扩散浓度会导致聚集,减少分子面积,并将膜的硬度提高到约0.30 m2/mg的临界阈值,超过该阈值就会形成多层膜,而不会进一步增加硬度。石油顶相的存在导致沥青烯烃侧链向石油方向组织,从而形成致密性较低、柔韧性较强的薄膜。相反,由于静电相互作用锚定沥青质分子,水相中的盐增加了界面刚性,揭示了油诱导的烃链组织和盐诱导的锚定之间的补偿效应。这些发现强调了使用简化的水-空气界面的局限性,并强调了盐水-油界面为研究沥青质的行为提供了更现实的模型。此外,对于控制沥青质膜的分子水平相互作用也提供了新的见解,这有助于理解复杂油气-水系统中的界面现象,其中盐度和界面类型都是关键变量。本文章由计算机程序翻译,如有差异,请以英文原文为准。
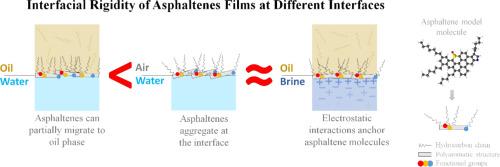
Relevance of brine-oil interfaces in understanding interfacial behavior of asphaltenes
Langmuir pressure-area (π-A) isotherms have been used to investigate the behavior of asphaltene interfacial films, specially at simple water-air interfaces. However, to understand their behavior in complex interfaces, such as those find in crude oil emulsions, more realistic interfaces are essential. Here, asphaltenes were characterized via SEM microscopy, EDS, CHN elemental analysis, and FTIR, and the behavior of their films was investigated at water-air, water-oil, and brine-oil interfaces using π-A isotherms and rheological measurements. Results reveal that the nature of the interface and asphaltene concentration dictate film rigidity and molecular organization. At the water-air, increasing the initial-spread concentration of asphaltenes leads to aggregation, reducing the molecular area and enhancing film rigidity up to a critical threshold of approximately 0.30 m2/mg, beyond which multilayer formation occurs without further increasing rigidity. The presence of oil top-phase induces asphaltene hydrocarbon side-chains to organize towards the oil, resulting in less compact, more flexible films. Conversely, salts in the aqueous phase increases interfacial rigidity due to electrostatic interactions that anchor asphaltene molecules, revealing a compensatory effect between oil-induced hydrocarbon chains organization and salt-induced anchoring. These findings underscore the limitations of using simplified water-air interfaces and highlight that brine-oil interfaces provide a more realistic model for studying asphaltene behavior. Also, novel insights into the molecular-level interactions governing asphaltene film are provided, which helps understanding the interfacial phenomena in complex hydrocarbon-water systems where both salinity and interface type are critical variables.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Molecular Liquids
化学-物理:原子、分子和化学物理
CiteScore
10.30
自引率
16.70%
发文量
2597
审稿时长
78 days
期刊介绍:
The journal includes papers in the following areas:
– Simple organic liquids and mixtures
– Ionic liquids
– Surfactant solutions (including micelles and vesicles) and liquid interfaces
– Colloidal solutions and nanoparticles
– Thermotropic and lyotropic liquid crystals
– Ferrofluids
– Water, aqueous solutions and other hydrogen-bonded liquids
– Lubricants, polymer solutions and melts
– Molten metals and salts
– Phase transitions and critical phenomena in liquids and confined fluids
– Self assembly in complex liquids.– Biomolecules in solution
The emphasis is on the molecular (or microscopic) understanding of particular liquids or liquid systems, especially concerning structure, dynamics and intermolecular forces. The experimental techniques used may include:
– Conventional spectroscopy (mid-IR and far-IR, Raman, NMR, etc.)
– Non-linear optics and time resolved spectroscopy (psec, fsec, asec, ISRS, etc.)
– Light scattering (Rayleigh, Brillouin, PCS, etc.)
– Dielectric relaxation
– X-ray and neutron scattering and diffraction.
Experimental studies, computer simulations (MD or MC) and analytical theory will be considered for publication; papers just reporting experimental results that do not contribute to the understanding of the fundamentals of molecular and ionic liquids will not be accepted. Only papers of a non-routine nature and advancing the field will be considered for publication.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


