磷酸盐与铀的萃取和聚集行为:来自DFT和EXAFS研究的见解
IF 5.2
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
磷酸盐基溶剂因其高碱度而被认为是从硝酸介质中分离锕系元素的重要萃取剂之一。研究了在硝酸介质中,以正十二烷(n-DD)为溶剂,用1.1 M的磷酸二丁基丁酯(DBBP)和磷酸二氨基酯(DAAP)溶液在303 K下从硝酸铀酰溶液中萃取U(VI)的行为。以DBBP和DAAP为溶剂,在交叉电流模式下对硝酸铀酰溶液和负载有机相中U(VI)的定量提取和剥离进行了级数评估。研究了以膦酸盐为溶剂,在交叉电流模式下从硝酸介质中定量提取U的方法。采用喷射混合器-沉淀装置,在1.1 M DAAP/n-DD的硝酸介质中对U溶液进行了连续逆流液-液萃取,考察了溶剂在连续模式下的性能。采用动态光散射(DLS)研究了有机相中U(VI)对团聚体粒径的影响。用EXAFS分析了铀与DBBP和DAAP的配合物,了解了铀与膦酸盐基溶剂的配位行为。利用扩展x射线吸收精细结构分析(EXAFS)计算了U配合物的配位数和键长。随后进行了DFT研究,以了解硝酸铀酰与配体之间的分子水平相互作用。进行了详细的能量分解分析(EDA)来揭示金属-配体相互作用的各种能量成分。溶剂萃取实验、DLS研究、EXAFS分析和DFT计算结果吻合较好。本文章由计算机程序翻译,如有差异,请以英文原文为准。
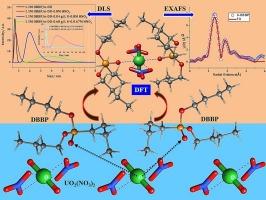
The extraction and aggregation behavior of phosphonates with uranium: Insights from DFT and EXAFS studies
Phosphonate based solvents are considered as one of the important extractants for the separation of actinides from nitric acid medium due to its high basicity. The extraction behavior of U(VI) by 1.1 M solution of Dibutylbutyl phosphonate (DBBP) and Diamylamyl phosphonate (DAAP) in n-dodecane (n-DD) from uranyl nitrate solutions in nitric acid medium has been investigated at 303 K. Number of stages were evaluated for the quantitative extraction and stripping of U(VI) from uranyl nitrate solutions and loaded organic phase using DBBP and DAAP based solvents in cross-current mode. Quantitative extraction of U from nitric acid medium was observed with phosphonate based solvents in cross-current mode. Continuous counter-current liquid-liquid solvent extraction runs were performed with U solutions in nitric acid medium with 1.1 M DAAP/n-DD using ejector mixer-settler facility and the performance of the solvent in continuous mode was investigated. Dynamic Light Scattering (DLS) studies were performed to understand the variation of aggregate size with U(VI) in the organic phase. EXAFS analysis of uranium complexes with DBBP and DAAP were performed to understand the coordination behavior of U with phosphonate based solvents. The coordination number of U and the bond lengths at which the atoms are bonded to U were computed from Extended X-ray Absorption Fine Structure analysis (EXAFS) of U complexes. Subsequently the DFT studies were also performed to understand the molecular level interaction between uranyl nitrate and the ligands. A detailed Energy Decomposition Analysis (EDA) was performed to unravel the various energy components of the metal-ligand interactions. A good agreement was observed between the solvent extraction experiments, DLS studies, EXAFS analysis and DFT computations.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Molecular Liquids
化学-物理:原子、分子和化学物理
CiteScore
10.30
自引率
16.70%
发文量
2597
审稿时长
78 days
期刊介绍:
The journal includes papers in the following areas:
– Simple organic liquids and mixtures
– Ionic liquids
– Surfactant solutions (including micelles and vesicles) and liquid interfaces
– Colloidal solutions and nanoparticles
– Thermotropic and lyotropic liquid crystals
– Ferrofluids
– Water, aqueous solutions and other hydrogen-bonded liquids
– Lubricants, polymer solutions and melts
– Molten metals and salts
– Phase transitions and critical phenomena in liquids and confined fluids
– Self assembly in complex liquids.– Biomolecules in solution
The emphasis is on the molecular (or microscopic) understanding of particular liquids or liquid systems, especially concerning structure, dynamics and intermolecular forces. The experimental techniques used may include:
– Conventional spectroscopy (mid-IR and far-IR, Raman, NMR, etc.)
– Non-linear optics and time resolved spectroscopy (psec, fsec, asec, ISRS, etc.)
– Light scattering (Rayleigh, Brillouin, PCS, etc.)
– Dielectric relaxation
– X-ray and neutron scattering and diffraction.
Experimental studies, computer simulations (MD or MC) and analytical theory will be considered for publication; papers just reporting experimental results that do not contribute to the understanding of the fundamentals of molecular and ionic liquids will not be accepted. Only papers of a non-routine nature and advancing the field will be considered for publication.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


