基于磺酰基试剂的烯类光驱动双官能化的机理研究:无催化剂方法
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
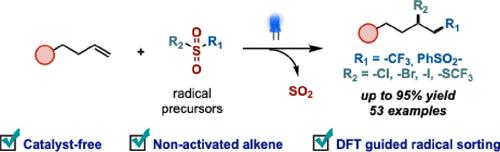
可见光介导的非活化烯烃双官能化为构建与药物化学、聚合物科学和精细化学品合成相关的多种分子框架提供了一种可持续和有效的策略。虽然现有的方法──如光氧化还原催化、能量转移(EnT)和配体到金属电荷转移(LMCT)──已经证明是成功的,但它们通常需要外部光催化剂来实现高反应活性。或者,电子供体-受体(EDA)配合物已经被探索,但这些方法通常依赖于特殊设计的底物,限制了这种反应的范围。为了克服这些限制,我们提出了一种dft引导的方法来识别光介导的非活化烯烃双官能化的合适自由基,消除了辅助催化剂或试剂的要求。我们的DFT计算阐明了一般的反应机制,并提供了对区域选择性的见解。此外,利用时间相关DFT (TD-DFT)计算模拟了紫外-可见光谱,并分析了关键光诱导跃迁所涉及的轨道,指导了合适光源的选择。我们进一步研究了生成的自由基的亲电性和亲核性,以预测其加成的区域选择性。本研究为设计不依赖光催化剂或底物特异性EDA配合物的原子经济双功能化反应提供了框架,并为该领域的进一步发展开辟了新的途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Mechanistic Insights into the Light-Driven Difunctionalization of Alkenes with a Sulfonyl-Based Reagent: A Catalyst-Free Approach
Visible-light-mediated difunctionalization of nonactivated alkenes offers a sustainable and efficient strategy for constructing diverse molecular frameworks relevant to medicinal chemistry, polymer science, and synthesis of fine chemicals. While established approaches─such as photoredox catalysis, energy transfer (EnT), and ligand-to-metal charge transfer (LMCT)─have demonstrated success, they typically require external photocatalysts to achieve high reactivity. Alternatively, electron donor–acceptor (EDA) complexes have been explored, but these methods often rely on specially designed substrates, limiting the scope of this reaction. To overcome these limitations, we present a DFT-guided approach for identifying suitable radicals for light-mediated difunctionalization of nonactivated alkenes, eliminating the requirement of auxiliary catalysts or reagents. Our DFT calculations elucidate general reaction mechanisms and provide insights into regioselectivity. Additionally, time-dependent DFT (TD-DFT) calculations are employed to simulate UV–vis spectra and analyze the orbitals involved in key photoinduced transitions, guiding the selection of appropriate light sources. We further investigated the electrophilic and nucleophilic properties of the generated radicals to predict the regioselectivity of their additions. This study provides a framework for designing atom-economical difunctionalization reactions without relying on photocatalysts or substrate-specific EDA complexes and opens new avenues for further development in this area.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


