羰基:一种电化学硫醚介导的醇氧化成醛和酮的物质
IF 7.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
我们报告了eCarbonyls,一种可扩展的,无金属的醇的电化学氧化,模仿经典的Swern反应的关键特征,同时避免了对低温条件和危险试剂的依赖。该工艺在室温下在未分裂的细胞中操作,采用稳定的硫醚介质产生活性自由基阳离子中间体,使伯醇和仲醇选择性氧化为醛和酮。该方法显示出广泛的底物范围,在超过25个例子中分离收率高达98%,并且对敏感官能基团(包括氮化化合物,硼酸盐和硅醚)具有良好的耐受性。机理研究证实了阳极生成的硫醚自由基阳离子的作用,并强调了外部碱的重要性。值得注意的是,eCarbonyls易于扩展和适应流动电解,可实现多图合成,并为学术和工业应用提供安全,可持续的平台。本文章由计算机程序翻译,如有差异,请以英文原文为准。
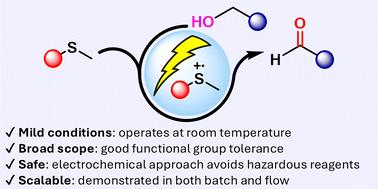
eCarbonyls: an electrochemical thioether mediated oxidation of alcohols to aldehydes and ketones
We report eCarbonyls, a scalable, metal-free electrochemical oxidation of alcohols that mimics key features of the classical Swern reaction while avoiding its reliance on cryogenic conditions and hazardous reagents. Operating at room temperature in an undivided cell, this process employs a stable thioether mediator to generate reactive radical cation intermediates that enable selective oxidation of primary and secondary alcohols to aldehydes and ketones. The method displays broad substrate scope, with up to 98% isolated yields across more than 25 examples, and excellent tolerance toward sensitive functional groups, including azides, boronates, and silyl ethers. Mechanistic studies confirm the role of anodically generated thioether radical cations and highlight the importance of the external base. Notably, eCarbonyls is readily scalable and adaptable to flow electrolysis, enabling multigram synthesis and offering a safe, sustainable platform for academic and industrial applications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Science
CHEMISTRY, MULTIDISCIPLINARY-
CiteScore
14.40
自引率
4.80%
发文量
1352
审稿时长
2.1 months
期刊介绍:
Chemical Science is a journal that encompasses various disciplines within the chemical sciences. Its scope includes publishing ground-breaking research with significant implications for its respective field, as well as appealing to a wider audience in related areas. To be considered for publication, articles must showcase innovative and original advances in their field of study and be presented in a manner that is understandable to scientists from diverse backgrounds. However, the journal generally does not publish highly specialized research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


