铜催化芳烃的不对称C-H磺化反应
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
作为一类重要的手性含硫产物,亚砜亚胺作为功能分子在各个领域发挥着重要的作用。本文报道了在铜催化下,杂环芳烃与亚砜胺经C-H氧化亚砜化反应高效合成亚砜胺。特别是,在温和的条件下,以空气或有机过氧化物作为氧化剂,吲哚和萘酚的亚胺化反应具有优异的区域和对映选择性,而亚胺化产物的构型不稳定性进一步凸显了该催化体系的挑战。实验和计算机制研究揭示了双氢原子转移(HAT)途径,C-S的形成是在八元环过渡态中通过外球HAT引发的自由基-自由基偶联与协调的芳烃HAT和C-S键形成的。本文章由计算机程序翻译,如有差异,请以英文原文为准。
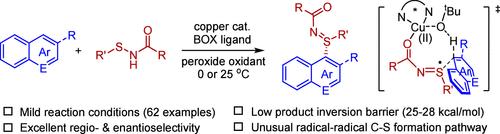
Copper-Catalyzed Asymmetric C–H Sulfilimination of Arenes via HAT-Primed C–S Radical–Radical Coupling
As an important class of chiral-at-sulfur products, sulfilimines play an important role in diverse fields as functional molecules. Reported herein is the efficient synthesis of sulfilimines via oxidative C–H sulfilimination of (hetero)arenes with sulfenamides under copper catalysis. In particular, the sulfilimination of indoles and naphthols proceeded under mild conditions in excellent regio- and enantioselectivity with air or organic peroxide as the oxidant, and the configurational lability of the sulfilimine products further highlighted the challenge of this catalytic system. Experimental and computational mechanistic studies revealed a dual hydrogen atom transfer (HAT) pathway, and the C–S formation occurs via an outer-sphere HAT-primed radical–radical coupling with concerted arene HAT and C–S bonding in an eight-membered ring transition state.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


