双核镁催化硼氢化:揭示金属-配体极性不饱和键的协同活化
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
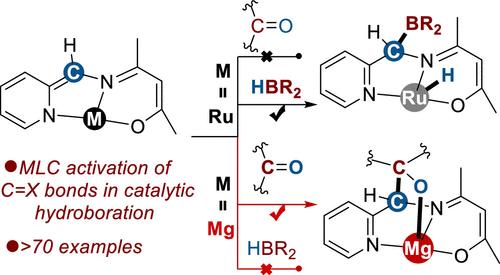
金属配体合作(MLC)已成为合成化学和生物化学中催化小分子活化的基石策略。虽然MLC在催化硼氢化方面取得了重大进展,但大多数已知的系统依赖于MLC介导的H-B键活化来产生金属氢化物中间体。相比之下,通过直接MLC激活极性不饱和键的催化硼氢化反应仍然很大程度上未被探索,尽管它有可能扩大底物范围并实现新的选择性。在这里,我们报道了一个双核镁配合物[Mg]-1,由能够在每个金属中心进行去芳构化-再芳构化MLC的重阴离子配体支撑。该复合物表现出非常规的MLC行为,优先激活酮类而不是HBpin,形成镁酮加合物[Mg]-2。这种选择性不同于具有相同配体框架的类似钌(II)配合物,后者优先激活HBpin而不是酮以产生氢化钌。这些发现强调了与过渡金属类似物相比,主族金属体系的独特MLC反应性。动力学研究和DFT计算表明,速率决定步骤涉及氢化物从HBpin转移到镁-酮加合物中的酮,这是通过两个Mg中心的协同活化实现的。该催化剂具有广泛的用途,可实现70多种极性不饱和底物的硼氢化,包括具有挑战性的类,如硝基化合物。值得注意的是,它还介导喹啉衍生物的选择性1,4-硼氢化,这是主基团催化剂很少实现的转化。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Dinuclear Magnesium-Catalyzed Hydroboration: Unveiling Metal–Ligand Cooperative Activation of Polar Unsaturated Bonds
Metal–ligand cooperation (MLC) has emerged as a cornerstone strategy for catalytic small molecule activation across both synthetic and biological chemistry. While MLC has driven significant progress in catalytic hydroboration, most known systems rely on MLC-mediated activation of the H–B bond to generate metal hydride intermediates. In contrast, catalytic hydroboration via direct MLC activation of polar unsaturated bonds remains largely unexplored, despite its potential for expanding substrate scope and achieving novel selectivity. Here, we report a dinuclear magnesium complex [Mg]-1, supported by dianionic ligands capable of dearomatization-rearomatization MLC at each metal center. This complex exhibits unconventional MLC behavior, preferentially activating ketones over HBpin to form a magnesium-ketone adduct [Mg]-2. This selectivity diverges from that of analogous Ruthenium(II) complexes bearing the same ligand framework, which preferentially activate HBpin over ketones to yield ruthenium hydride species. These findings underscore the distinctive MLC reactivity of main-group metal systems compared to transition metal analogues. Kinetic studies and DFT calculations reveal that the rate-determining step involves hydride transfer from HBpin to the ketone within the magnesium–ketone adduct, enabled by synergistic activation across both Mg centers. The catalyst demonstrates broad utility, enabling the hydroboration of over 70 polar unsaturated substrates, including challenging classes such as nitro compounds. Notably, it also mediates selective 1,4-hydroboration of quinoline derivatives, a transformation rarely achieved with main-group catalysts.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


