绕过异常的奇奇巴宾反应的死端,提供了一种仿生学途径来获得前豪胺
Organic chemistry frontiers : an international journal of organic chemistry
Pub Date : 2025-09-05
DOI:10.1039/d5qo01111f
引用次数: 0
摘要
藻胺是一种高度受限的海洋生物碱,具有独特的自然骨架。这类生物碱的高度复杂性引发了有关其化学组装的问题。在本文中,我们提出了一个仿生场景,通过对经典奇奇巴宾吡啶合成的精细研究,特别是在其“异常”氧化版本中,实验证实了这一点。精细调整的还原条件和机械研究允许简明地注意到一种先进的和具有挑战性的中间体,我们称之为“预豪胺”。本文章由计算机程序翻译,如有差异,请以英文原文为准。
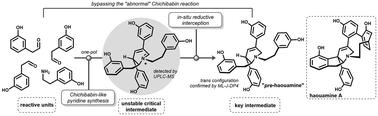
Bypassing the abnormal Chichibabin reaction dead-end provides a biomimetic access to pre-haouamine
Haouamines are highly constrained marine alkaloids possessing a unique in nature skeleton. The high degree of complexity of such alkaloids raises questions about their chemical assembly. This is addressed in this paper in which we propose a biomimetic scenario corroborated experimentally by a fine study of the classical Chichibabin pyridine synthesis, especially in its “abnormal” oxidative version. Finely tuned reductive conditions and mechanistic investigations permit the concise obtention of an advanced and challenging intermediate that we coined “pre-haouamine”.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.80
自引率
0.00%
发文量
0

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


