镭对骨微环境的影响
IF 14.6
1区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
镭-223 (223Ra)是一种寻骨、释放α粒子的放射性核素,已被批准用于治疗转移性前列腺癌患者,目前正在临床试验中对原发性和转移性骨癌进行测试。ra在骨周转率高的矿化骨区积累,由于α粒子的组织外显率较短,其影响仅限于骨髓界面100 μm内。最近的一项临床研究表明,223Ra的使用显著增加了骨折率,主要是在无肿瘤的骨骼中。重要的是,这种骨骼脆弱的生物学机制尚不清楚。在这项工作中,我们将显微计算机断层扫描和力学研究与基于三维荧光显微镜的离体空间生物学分析相结合,以阐明223Ra对骨和关键骨基质细胞成分的影响。我们发现223Ra引起了严重的骨小梁丢失,但对皮质骨没有可检测到的影响。此外,223Ra损伤成骨细胞成骨活性,这与破骨细胞数量的短暂增加和长期脂肪细胞形成相平行。总的来说,这些结果表明,223Ra对骨骼健康的影响是由多种骨基质细胞成分协调的。223Ra介导的骨小梁丢失可通过唑来膦酸预防,唑来膦酸应始终与223Ra联合使用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
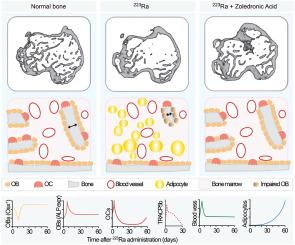
Dissecting the effects of 223Radium on the bone microenvironment
Radium-223 (223Ra) is a bone-seeking, alpha-particle-emitting radionuclide that is approved for the treatment of patients with metastatic prostate cancer and is currently being tested in clinical trials for primary and metastatic cancers to the bone. 223Ra accumulates in mineralized bone areas with high bone turnover, where its effects are confined within 100 μm of the bone–marrow interface due to the short tissue penetrance of the alpha particles. A recent clinical study has shown a significantly increased fracture rate associated with the administration of 223Ra, mostly in tumor-free bones. Importantly, the biological mechanisms underlying this bone fragility remain unclear. In this work, we combined micro-computed tomography and mechanical studies with ex vivo spatial biology analysis based on 3D fluorescence microscopy to clarify the effects of 223Ra on bone and key bone stromal cell components. We found that 223Ra caused major trabecular bone loss with no detectable impact on cortical bone. In addition, 223Ra impaired osteoblast bone-forming activity, which was paralleled by a transient increase in osteoclast number and long-term adipocyte formation. Overall, these results suggest that the impact of 223Ra on bone health is orchestrated by multiple bone stromal cell components. 223Ra-mediated trabecular bone loss was prevented by administration of zoledronic acid, which should always be combined with 223Ra.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Acta Pharmaceutica Sinica. B
Pharmacology, Toxicology and Pharmaceutics-General Pharmacology, Toxicology and Pharmaceutics
CiteScore
22.40
自引率
5.50%
发文量
1051
审稿时长
19 weeks
期刊介绍:
The Journal of the Institute of Materia Medica, Chinese Academy of Medical Sciences, and the Chinese Pharmaceutical Association oversees the peer review process for Acta Pharmaceutica Sinica. B (APSB).
Published monthly in English, APSB is dedicated to disseminating significant original research articles, rapid communications, and high-quality reviews that highlight recent advances across various pharmaceutical sciences domains. These encompass pharmacology, pharmaceutics, medicinal chemistry, natural products, pharmacognosy, pharmaceutical analysis, and pharmacokinetics.
A part of the Acta Pharmaceutica Sinica series, established in 1953 and indexed in prominent databases like Chemical Abstracts, Index Medicus, SciFinder Scholar, Biological Abstracts, International Pharmaceutical Abstracts, Cambridge Scientific Abstracts, and Current Bibliography on Science and Technology, APSB is sponsored by the Institute of Materia Medica, Chinese Academy of Medical Sciences, and the Chinese Pharmaceutical Association. Its production and hosting are facilitated by Elsevier B.V. This collaborative effort ensures APSB's commitment to delivering valuable contributions to the pharmaceutical sciences community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


