自发光脂质体原位触发光动力疗法联合放射性核素疗法协同治疗肺癌
IF 14.6
1区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
肺癌持续的高患病率和较差的生存结果强调了对创新治疗方式的迫切需要。在这里,我们提出了一种新的多功能输送平台,用于协同治疗肺部恶性肿瘤,将原位触发光动力治疗(PDT)与放疗相结合。新平台CLL是通过将一种新的活性氧(ROS)可触发光敏剂鲁米诺共轭氯e6 (Ce6)加载到脂质体中而开发的。CLL可以在氧化应激下通过生物发光共振能量转移效应被激活,从而产生单线态氧,无需外照射即可靶向治疗肿瘤。体外研究显示,CLL对4T1和A549肿瘤细胞均有显著的细胞毒性作用。此外,通过将放射性核素177Lu加入到CLL中,设计了pdt -放射性药物联合纳米疗法CLL-177Lu。CLL-177Lu在4T1和A549肿瘤细胞以及4T1乳腺癌肺转移或A549肿瘤异种移植小鼠模型中显示出协同抗肿瘤作用。机制上,CLL-177Lu可诱导单线态氧/ROS生成,增强肿瘤细胞凋亡,促进M1巨噬细胞介导的免疫治疗。初步评估显示CLL-177Lu具有良好的特性,突出了其作为一种有前景的癌症纳米疗法的潜力。此外,CLL可以作为提供一系列疗法的通用平台,以实现协同抗肿瘤效果。本文章由计算机程序翻译,如有差异,请以英文原文为准。
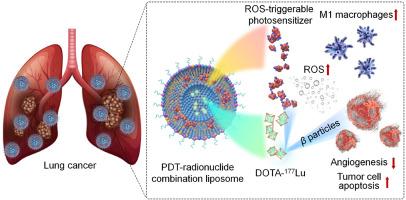
Self-illuminating liposome-derived in situ triggerable photodynamic therapy combining radionuclide therapy for synergistic treatment of lung cancer
The persistent high prevalence and poor survival outcomes of lung cancer underscore the urgent need for innovative therapeutic modalities. Here, we present a novel multifunctional delivery platform for the synergistic treatment of lung malignancies, combining in situ-triggerable photodynamic therapy (PDT) with radiotherapy. The new platform CLL was developed by loading a new reactive oxygen species (ROS)-triggerable photosensitizer, luminol-conjugated chlorin e6 (Ce6), into liposomes. CLL can be activated through the bioluminescence resonance energy transfer effect under oxidative stress, thereby producing singlet oxygen for targeted tumor treatment without external irradiation. In vitro studies showed significant cytotoxic effects of CLL in both 4T1 and A549 tumor cells. Furthermore, a PDT-radiopharmaceutical combination nanotherapy CLL-177Lu was engineered by incorporating the radionuclide 177Lu into CLL. CLL-177Lu demonstrated synergistic antitumor effects in 4T1 and A549 tumor cells, as well as in mouse models of 4T1 breast cancer lung metastasis or A549 tumor xenografts. Mechanistically, CLL-177Lu can induce singlet oxygen/ROS generation, enhance tumor cell apoptosis, and promote M1 macrophage-mediated immunotherapy. Preliminary assessments showed a favorable profile for CLL-177Lu, highlighting its potential as a promising nanotherapy for cancer treatment. Additionally, CLL can serve as a versatile platform for delivering a range of therapies to achieve synergistic antitumor effects.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Acta Pharmaceutica Sinica. B
Pharmacology, Toxicology and Pharmaceutics-General Pharmacology, Toxicology and Pharmaceutics
CiteScore
22.40
自引率
5.50%
发文量
1051
审稿时长
19 weeks
期刊介绍:
The Journal of the Institute of Materia Medica, Chinese Academy of Medical Sciences, and the Chinese Pharmaceutical Association oversees the peer review process for Acta Pharmaceutica Sinica. B (APSB).
Published monthly in English, APSB is dedicated to disseminating significant original research articles, rapid communications, and high-quality reviews that highlight recent advances across various pharmaceutical sciences domains. These encompass pharmacology, pharmaceutics, medicinal chemistry, natural products, pharmacognosy, pharmaceutical analysis, and pharmacokinetics.
A part of the Acta Pharmaceutica Sinica series, established in 1953 and indexed in prominent databases like Chemical Abstracts, Index Medicus, SciFinder Scholar, Biological Abstracts, International Pharmaceutical Abstracts, Cambridge Scientific Abstracts, and Current Bibliography on Science and Technology, APSB is sponsored by the Institute of Materia Medica, Chinese Academy of Medical Sciences, and the Chinese Pharmaceutical Association. Its production and hosting are facilitated by Elsevier B.V. This collaborative effort ensures APSB's commitment to delivering valuable contributions to the pharmaceutical sciences community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


