mRNA显示使发现邻近触发的共价肽-药物偶联物
IF 14.6
1区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
肽-药物偶联物(PDCs)在精确肿瘤学中已经成为一种很有前途的模式,能够靶向递送细胞毒性有效载荷,同时最大限度地减少脱靶毒性。基于硫(VI)氟交换(SuFEx)化学的共价战斗部的整合,增加了药物靶点停留时间和肿瘤蓄积。然而,现有的共价肽(CP)文库筛选方法需要翻译后战斗部偶联,限制了通量。在这里,我们提出了一个集成的mRNA展示平台,该平台在核糖体合成过程中包含共价弹头,能够有效筛选超多样化的共价大环肽库(1013个变体)。该方法利用n-氯乙酰-d-苯丙氨酸和氟硫酸盐- L -酪氨酸的位点特异性结合,通过邻近触发SuFEx加速了不可逆结合(Ki = 3.58 μmol/L) nectin -4靶向肽CP-N1-N3的发现。该肽进一步与细胞毒载荷偶联,得到共价PDC CP-N1-MMAE,对MDA-MB-468细胞具有强细胞毒性(IC50≈43 nmol/L)。该平台为共价药物的精准发现建立了新的范式。本文章由计算机程序翻译,如有差异,请以英文原文为准。
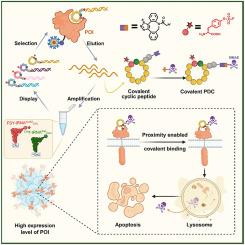
mRNA display-enabled discovery of proximity-triggered covalent peptide–drug conjugates
Peptide–drug conjugates (PDCs) have emerged as a promising modality in precision oncology, enabling targeted delivery of cytotoxic payloads while minimizing off-target toxicity. The integration of covalent warheads, such as those based on sulfur(VI) fluoride exchange (SuFEx) chemistry, enhances drug–target residence time and tumor accumulation. However, existing screening methods for covalent peptide (CP) libraries require post-translational warhead conjugation, limiting throughput. Here, we present an integrated mRNA display platform that incorporates covalent warheads during ribosomal synthesis, enabling efficient screening of ultra-diverse covalent macrocyclic peptide libraries (>1013 variants). This approach, using site-specific incorporation of N-chloroacetyl-d-phenylalanine and fluorosulfate-l-tyrosine, accelerated the discovery of irreversibly binding (Ki = 3.58 μmol/L) Nectin-4-targeting peptide CP-N1-N3 via proximity-triggered SuFEx. The peptide was further conjugated to cytotoxic payloads, yielding the covalent PDC CP-N1-MMAE with potent cytotoxicity (IC50 ≈ 43 nmol/L) against MDA-MB-468 cells. This platform establishes a new paradigm for precision covalent drug discovery.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Acta Pharmaceutica Sinica. B
Pharmacology, Toxicology and Pharmaceutics-General Pharmacology, Toxicology and Pharmaceutics
CiteScore
22.40
自引率
5.50%
发文量
1051
审稿时长
19 weeks
期刊介绍:
The Journal of the Institute of Materia Medica, Chinese Academy of Medical Sciences, and the Chinese Pharmaceutical Association oversees the peer review process for Acta Pharmaceutica Sinica. B (APSB).
Published monthly in English, APSB is dedicated to disseminating significant original research articles, rapid communications, and high-quality reviews that highlight recent advances across various pharmaceutical sciences domains. These encompass pharmacology, pharmaceutics, medicinal chemistry, natural products, pharmacognosy, pharmaceutical analysis, and pharmacokinetics.
A part of the Acta Pharmaceutica Sinica series, established in 1953 and indexed in prominent databases like Chemical Abstracts, Index Medicus, SciFinder Scholar, Biological Abstracts, International Pharmaceutical Abstracts, Cambridge Scientific Abstracts, and Current Bibliography on Science and Technology, APSB is sponsored by the Institute of Materia Medica, Chinese Academy of Medical Sciences, and the Chinese Pharmaceutical Association. Its production and hosting are facilitated by Elsevier B.V. This collaborative effort ensures APSB's commitment to delivering valuable contributions to the pharmaceutical sciences community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


