靶向递送BMPR2 mRNA通过逆转肺血管重构来减轻肺动脉高压
IF 14.6
1区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
内皮细胞中骨形态发生蛋白2型受体(BMPR2)信号传导中断驱动肺动脉高压(PAH)。然而,通过脂质纳米颗粒(LNPs)靶向恢复这一信号通路尚未被探索作为一种治疗方法。在这里,我们采用实验设计来优化我们实验室开发的针对肺内皮细胞的LNPs的递送效率,结果在不含辅助脂质的简化三组分配方中,LNPs的递送效率显著提高35倍。BMPR2 mRNA LNPs通过逆转肺血管重构,有效逆转了两种实验大鼠模型(单罗林或su5416 -缺氧)中建立的PAH。具体来说,BMPR2 mRNA LNPs补充了BMPR2蛋白的表达,随后激活了下游途径,p-SMAD1/5/9和ID1蛋白水平升高证实了这一点。肺动脉闭塞的缓解表现为肺动脉中膜变薄,全肌化血管比例减少。富尔顿指数下降、右心室心肌细胞横截面积下降、胶原沉积减少表明右心室肥厚减轻。肺动脉血流加速时间/肺动脉血流射血时间比值的增加证明右心室功能的有效恢复。这些发现强调了通过肺内皮细胞特异性LNPs恢复BMPR2信号通路治疗PAH的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
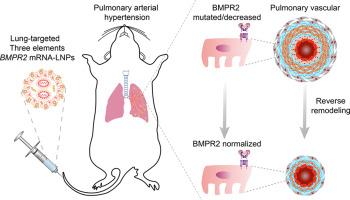
Targeted delivery of BMPR2 mRNA attenuates pulmonary arterial hypertension by reversing pulmonary vascular remodeling
Disrupted bone morphogenetic protein type 2 receptor (BMPR2) signaling in endothelial cells drives pulmonary arterial hypertension (PAH). However, targeted recovery of this signaling pathway by lipid nanoparticles (LNPs) has not been explored as a therapy. Here, we employed Design of Experiments to optimize the delivery efficiency of LNPs targeting pulmonary endothelial cells developed by our laboratory, resulting in a remarkable 35-fold increase in a simplified three-component formulation without helper lipids. Administration of BMPR2 mRNA LNPs effectively reversed established PAH in two experimental rat models (monocrotaline or SU5416-hypoxia) by reversing pulmonary vascular remodeling. Specifically, BMPR2 mRNA LNPs replenished the expression of BMPR2 protein and subsequently activated downstream pathways, as confirmed by elevated levels of p-SMAD1/5/9 and ID1 proteins. The relief of pulmonary arterial occlusion was demonstrated by thinned pulmonary arterial media and decreased proportion of full muscularized vessels. Alleviation of right ventricular hypertrophy was indicated by declined Fulton index, the cross-sectional area of right ventricular cardiomyocytes as well as collagen deposition. Effective recovery of right ventricular function was evidenced by increased pulmonary artery flow acceleration time/pulmonary artery flow ejection time ratio. These findings underscore the potential of restoring BMPR2 signaling through pulmonary endothelial cell-specific LNPs for treating PAH.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Acta Pharmaceutica Sinica. B
Pharmacology, Toxicology and Pharmaceutics-General Pharmacology, Toxicology and Pharmaceutics
CiteScore
22.40
自引率
5.50%
发文量
1051
审稿时长
19 weeks
期刊介绍:
The Journal of the Institute of Materia Medica, Chinese Academy of Medical Sciences, and the Chinese Pharmaceutical Association oversees the peer review process for Acta Pharmaceutica Sinica. B (APSB).
Published monthly in English, APSB is dedicated to disseminating significant original research articles, rapid communications, and high-quality reviews that highlight recent advances across various pharmaceutical sciences domains. These encompass pharmacology, pharmaceutics, medicinal chemistry, natural products, pharmacognosy, pharmaceutical analysis, and pharmacokinetics.
A part of the Acta Pharmaceutica Sinica series, established in 1953 and indexed in prominent databases like Chemical Abstracts, Index Medicus, SciFinder Scholar, Biological Abstracts, International Pharmaceutical Abstracts, Cambridge Scientific Abstracts, and Current Bibliography on Science and Technology, APSB is sponsored by the Institute of Materia Medica, Chinese Academy of Medical Sciences, and the Chinese Pharmaceutical Association. Its production and hosting are facilitated by Elsevier B.V. This collaborative effort ensures APSB's commitment to delivering valuable contributions to the pharmaceutical sciences community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


