抗cd24抗体-一氧化氮供体偶联物携带自生物正交可切割连接
IF 14.6
1区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
三阴性乳腺癌(TNBC)是一种高度侵袭性的恶性肿瘤,主要通过化疗治疗。我们的临床样本分析显示,TNBC肿瘤细胞中CD24表达升高与患者生存率之间存在显著相关性。我们开发了一种新的抗体-药物偶联物(ADC),命名为HN03,由一种带有工程半胱氨酸的抗体组成,通过新型Pt(IV)介导的生物正交自切割连接体,与低毒一氧化氮(NO)前体作为有效载荷进行位点特异性偶联。HN03特异性靶向表达高水平CD24的肿瘤细胞,同时产生顺铂并在激活后释放NO。HN03在体外和体内均表现出较强的抗肿瘤活性。不同剂量均可显著抑制肿瘤生长,防止肿瘤转移,毒性明显低于传统化疗药物。我们发现其作用的关键机制涉及诱导细胞凋亡和内质网应激,显著减少m2型巨噬细胞的数量。总的来说,HN03作为TNBC的一种有前景的治疗选择脱颖而出,提供了一种具有减少副作用和改善预后潜力的靶向治疗。此外,在连接器中使用Pt(IV)和NO前体作为有效载荷,增强了抗体-NO供体偶联物(ANC)的多功能性,为下一代adc的设计提供了新的途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
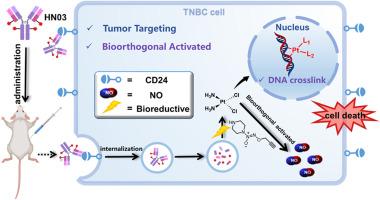
Anti-CD24 antibody-nitric oxide donor conjugates bearing a self-bioorthogonal cleavable linker
Triple-negative breast cancer (TNBC) is a highly aggressive malignancy predominantly managed via chemotherapy. Our clinical sample analysis revealed a significant correlation between elevated CD24 expression in TNBC tumor cells and patient survival rates. We developed a novel antibody–drug conjugate (ADC), named HN03, consisting of an antibody with engineered cysteines for site-specific conjugation with a low toxic nitric oxide (NO) precursor as its payload through a novel Pt(IV)-mediated bioorthogonal self-cleavable linker. HN03 specifically targets tumor cells expressing high levels of CD24, concurrently generating cisplatin and releasing NO upon activation. HN03 also exhibited potent in vitro and in vivo antitumor activity. It significantly reduced tumor growth at various doses, prevented tumor metastasis, with markedly lower toxicity than traditional chemotherapy agents. We found that a key mechanism of its action involved inducing apoptosis and endoplasmic reticulum stress, substantially decreasing the number of M2-type macrophages. Overall, HN03 stands out as a promising therapeutic option for TNBC, offering a targeted treatment with reduced side effects and the potential for improved outcomes. Furthermore, using Pt(IV) in the linker and an NO precursor as the payload enhances the versatility of the Antibody-NO donor Conjugate (ANC), offering new avenues for the design of the next generation of ADCs.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Acta Pharmaceutica Sinica. B
Pharmacology, Toxicology and Pharmaceutics-General Pharmacology, Toxicology and Pharmaceutics
CiteScore
22.40
自引率
5.50%
发文量
1051
审稿时长
19 weeks
期刊介绍:
The Journal of the Institute of Materia Medica, Chinese Academy of Medical Sciences, and the Chinese Pharmaceutical Association oversees the peer review process for Acta Pharmaceutica Sinica. B (APSB).
Published monthly in English, APSB is dedicated to disseminating significant original research articles, rapid communications, and high-quality reviews that highlight recent advances across various pharmaceutical sciences domains. These encompass pharmacology, pharmaceutics, medicinal chemistry, natural products, pharmacognosy, pharmaceutical analysis, and pharmacokinetics.
A part of the Acta Pharmaceutica Sinica series, established in 1953 and indexed in prominent databases like Chemical Abstracts, Index Medicus, SciFinder Scholar, Biological Abstracts, International Pharmaceutical Abstracts, Cambridge Scientific Abstracts, and Current Bibliography on Science and Technology, APSB is sponsored by the Institute of Materia Medica, Chinese Academy of Medical Sciences, and the Chinese Pharmaceutical Association. Its production and hosting are facilitated by Elsevier B.V. This collaborative effort ensures APSB's commitment to delivering valuable contributions to the pharmaceutical sciences community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


