肿瘤抑制子PPP2R1A K541被HAT1琥珀酰化,从而逆转糖异生/脂肪生成重塑的调节作用,从而显示癌基因功能
IF 14.6
1区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
代谢重编程在肿瘤中起着核心作用。然而,调节糖异生/脂肪生成重编程的关键驱动因素尚不清楚。在这里,我们试图确定组蛋白乙酰转移酶1 (HAT1)赋予肝癌糖异生/脂肪生成重编程的机制。二乙基亚硝胺(DEN)/四氯化碳(CCl4)诱导的肝癌在hat1基因敲除小鼠中几乎没有观察到。多组学研究发现,HAT1调节肝脏糖异生和脂肪生成。蛋白磷酸酶2支架亚单位α (PPP2R1A)通过磷酸烯醇丙酮酸羧激酶1 (PCK1)丝氨酸90去磷酸化促进糖异生,抑制脂肪生成,抑制肿瘤生长。HAT1在赖氨酸541 (K541)位点琥珀酰化PPP2R1A,阻断蛋白磷酸酶2A (PP2A)全酶的组装和与PCK1的相互作用,从而抑制PCK1的去磷酸化。hat1 -丁二酰化PPP2R1A通过PCK1丝氨酸90磷酸化参与糖异生/脂肪生成的重塑,抑制糖异生酶活性,激活甾醇调节元件结合蛋白1 (SREBP1)核积累诱导的脂肪生成基因表达,促进肿瘤生长。综上所述,HAT1对PPP2R1A赖氨酸541的丁二酰化通过PCK1 S90磷酸化逆转了糖异生/脂肪生成重塑的调节作用,从而支持肝癌。我们的发现为翻译后修饰(PTMs)将肿瘤抑制功能转化为致癌基因的机制提供了新的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
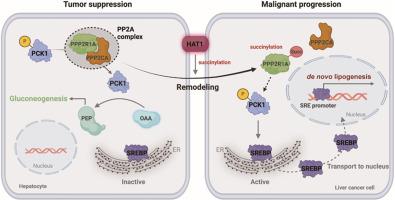
Succinylation of tumor suppressor PPP2R1A K541 by HAT1 converses the role in modulation of gluconeogenesis/lipogenesis remodeling to display oncogene function
Metabolic reprogramming plays a central role in tumors. However, the key drivers modulating reprogramming of gluconeogenesis/lipogenesis are poorly understood. Here, we try to identify the mechanism by which histone acetyltransferase 1 (HAT1) confers reprogramming of gluconeogenesis/lipogenesis in liver cancer. Diethylnitrosamine (DEN)/carbon tetrachloride (CCl4)-induced hepatocarcinogenesis was hardly observed in HAT1-knockout mice. Multi-omics identified that HAT1 modulated gluconeogenesis and lipogenesis in liver. Protein phosphatase 2 scaffold subunit alpha (PPP2R1A) promoted gluconeogenesis and inhibited lipogenesis by phosphoenolpyruvate carboxykinase 1 (PCK1) serine 90 dephosphorylation to suppress the tumor growth. HAT1 succinylated PPP2R1A at lysine 541 (K541) to block the assembly of protein phosphatase 2A (PP2A) holoenzyme and interaction with PCK1, resulting in the depression of dephosphorylation of PCK1. HAT1-succinylated PPP2R1A contributed to the remodeling of gluconeogenesis/lipogenesis by PCK1 serine 90 phosphorylation, leading to the inhibition of gluconeogenic enzyme activity and activating sterol regulatory element-binding protein 1 (SREBP1) nuclear accumulation-induced lipogenesis gene expression, which enhanced the tumor growth. In conclusion, succinylation of PPP2R1A lysine 541 by HAT1 converses the role in modulation of gluconeogenesis/lipogenesis remodeling through PCK1 S90 phosphorylation to support liver cancer. Our finding provides new insights into the mechanism by which post-translational modifications (PTMs) confer the conversion of tumor suppressor function to oncogene.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Acta Pharmaceutica Sinica. B
Pharmacology, Toxicology and Pharmaceutics-General Pharmacology, Toxicology and Pharmaceutics
CiteScore
22.40
自引率
5.50%
发文量
1051
审稿时长
19 weeks
期刊介绍:
The Journal of the Institute of Materia Medica, Chinese Academy of Medical Sciences, and the Chinese Pharmaceutical Association oversees the peer review process for Acta Pharmaceutica Sinica. B (APSB).
Published monthly in English, APSB is dedicated to disseminating significant original research articles, rapid communications, and high-quality reviews that highlight recent advances across various pharmaceutical sciences domains. These encompass pharmacology, pharmaceutics, medicinal chemistry, natural products, pharmacognosy, pharmaceutical analysis, and pharmacokinetics.
A part of the Acta Pharmaceutica Sinica series, established in 1953 and indexed in prominent databases like Chemical Abstracts, Index Medicus, SciFinder Scholar, Biological Abstracts, International Pharmaceutical Abstracts, Cambridge Scientific Abstracts, and Current Bibliography on Science and Technology, APSB is sponsored by the Institute of Materia Medica, Chinese Academy of Medical Sciences, and the Chinese Pharmaceutical Association. Its production and hosting are facilitated by Elsevier B.V. This collaborative effort ensures APSB's commitment to delivering valuable contributions to the pharmaceutical sciences community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


