(±)-Larutienine B和(±)-Melokhanine B、D、E、F和H的全合成
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
我们报道了一种通用的、可扩展的策略,在13-15步中简明地合成了6种c2 -螺菌吲哚生物碱。这种合成方法的特点是一个氧化的aza-Diels-Alder环加成反应和一个逆曼尼希/曼尼希反应来构建四环环体系,并在邻基参与下形成正式的迈克尔加成来安装侧链。值得注意的是,首次完成了月桂嘌呤B的全合成。概述的策略为解决合成乙烷类生物碱的立体控制困难提供了补充解决方案。本文章由计算机程序翻译,如有差异,请以英文原文为准。
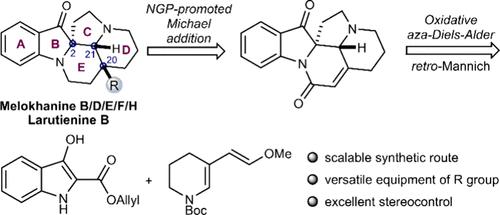
Total Synthesis of (±)-Larutienine B and (±)-Melokhanine B, D, E, F, and H
We report a versatile and scalable strategy for the concise syntheses of six C2-spirooxindole alkaloids in 13–15 steps. This synthetic approach features an oxidative aza-Diels–Alder cycloaddition and a retro-Mannich/Mannich reaction to build the tetracyclic ring system alongside a formal Michael addition facilitated by neighboring group participation to install the side chain. Notably, the first total synthesis of larutienine B has been accomplished. The outlined strategy offers a complementary solution for addressing the stereocontrol difficulties in syntheses of eburnane-type alkaloids.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


