具有大矫顽力场的四氮杂萘自由基桥接镝单分子磁体
IF 7.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
产生强磁耦合是设计多核镧系配合物的一个基本挑战。价态4f轨道固有的收缩性质使镧系元素无法与闭壳配体形成共价键。开壳桥接配体的使用使得弥散自由基自旋轨道与镧系元素的4f壳层有效地相互作用。本文将氮杂蒽配体1,4,5,8-四氮杂萘(tan)引入稀土化学。首先,我们通过Cp*2DyBPh4和K2(tan)的盐还原反应合成了含有抗磁性tan2 -桥的[(Cp*2Dy)2(μ-tan)] (1, Cp* =五甲基环戊二烯)。其次,我们将1化学氧化为[(Cp*2Dy)2(μ-tan˙)][BArF20](2),形成一个tan1−˙自由基桥。2是一种罕见的自由基桥接单分子磁体(SMM),其开放的磁滞环低于3.75 K, 1.8 K时的最大矫顽力场(HC)为1.373 T,这代表了一个值得注意的记录,因为与所有已知的有机自由基桥固有的双核镧系SMM相比,HC大约是其两倍。在低温下,tan1−˙/tan2−和DyIII/DyII氧化还原电位的紧密匹配可能是令人印象深刻的磁滞回线的起源,而高温下的磁行为可能受到自旋声子耦合的影响。通过先前的计算提出了将配体的还原电位与金属离子匹配以放大磁耦合的概要设计策略,但在本研究中首次得到实验证实。总之,高度可调的氮杂环自由基不仅在含自由基的smm中具有巨大的潜力,而且在高性能磁性材料中也具有巨大的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
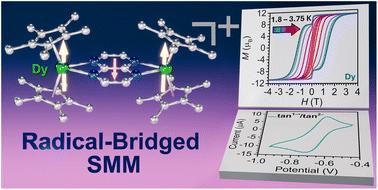
A tetraazanaphthalene radical-bridged dysprosium single-molecule magnet with a large coercive field
Generating strong magnetic coupling poses a fundamental challenge in the design of multinuclear lanthanide complexes. The inherently contracted nature of the valence 4f orbitals precludes the lanthanides from engaging in covalent bonding with closed-shell ligands. The employment of open-shell bridging ligands instead allows efficient interaction of the diffuse radical spin orbitals with the 4f shell of the lanthanides. Herein, we introduce the azaacene ligand, 1,4,5,8-tetraazanaphthalene (tan), into rare earth chemistry: first, we synthesized [(Cp*2Dy)2(μ-tan)] (1, Cp* = pentamethylcyclopentadienyl) containing a diamagnetic tan2− bridge from a salt metathesis reaction of Cp*2DyBPh4 and K2(tan). Second, we chemically oxidised 1 to [(Cp*2Dy)2(μ-tan˙)][BArF20] (2) comprising a tan1−˙ radical bridge. 2 is a rare radical-bridged single-molecule magnet (SMM) with open hysteresis loops below 3.75 K with a maximum coercive field (HC) of 1.373 T at 1.8 K, which represents a notable record as HC is approximately doubled compared to all known dinuclear lanthanide SMMs innate to organic radical bridges. A close match of the tan1−˙/tan2− and DyIII/DyII redox potentials may be the origin for the impressive hysteresis loops at low temperatures, while the magnetic behaviour at higher temperatures is likely impacted from spin–phonon coupling. The outlined design strategy of matching reduction potentials of the ligand with the metal ions to amplify magnetic coupling, was proposed via prior computations, but is within this study for the first time experimentally confirmed. In sum, highly-tunable azaacene radicals have immense potential not only for radical-containing SMMs but for high-performance magnetic materials at large.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Science
CHEMISTRY, MULTIDISCIPLINARY-
CiteScore
14.40
自引率
4.80%
发文量
1352
审稿时长
2.1 months
期刊介绍:
Chemical Science is a journal that encompasses various disciplines within the chemical sciences. Its scope includes publishing ground-breaking research with significant implications for its respective field, as well as appealing to a wider audience in related areas. To be considered for publication, articles must showcase innovative and original advances in their field of study and be presented in a manner that is understandable to scientists from diverse backgrounds. However, the journal generally does not publish highly specialized research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


