跨膜通道样4 (TMC4)可作为KCNQ1 (Kv7.1)钾通道的负调控因子。
IF 2.5
3区 生物学
Q3 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
TMC4是跨膜通道样(TMC)蛋白家族的成员。在这个家族中,TMC1和TMC2被认为在内耳形成机电转导(MET)通道。另一方面,其他TMC家族成员(TMC3-8)的内在功能很大程度上是未知的。KCNQ1 (Kv7.1)通道是电压门控钾通道,其辅助亚基KCNE蛋白(如KCNQ1/KCNE1复合体参与心脏复极化,KCNQ1/KCNE3复合体参与上皮离子运输)在生理上发挥重要作用。最近有报道称,TMC1和TMC2与KCNQ1相互作用,抑制KCNQ1的K+电流。然而,KCNQ1与其他TMC蛋白之间的关系尚未被研究。在这里,我们展示了过表达的TMC4和KCNQ1之间的一种新的相互作用和功能关联。head Halo试验和FRET分析揭示了这两种蛋白之间的物理相互作用。全细胞膜片钳记录显示,TMC4的共表达降低了KCNQ1电流密度,但没有改变它们的电压依赖性和激活动力学。在KCNQ1/KCNE1和KCNQ1/KCNE3通道复合物中也观察到这种效应。利用AlphaFold-Multimer进行结构预测,发现TMC4和KCNQ1之间可能存在相互作用位点。诱变后膜片钳记录表明,这些位点上的特定氨基酸残基有助于TMC4的抑制作用。这些结果表明,TMC4可以作为KCNQ1通道的负调节因子。我们的发现可以加深对KCNQ1通道调控的认识,并为TMC4在各种生理和病理条件下的功能提供潜在的研究方向。本文章由计算机程序翻译,如有差异,请以英文原文为准。
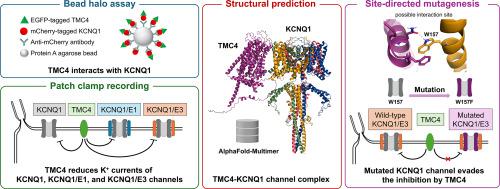
Transmembrane channel-like 4 (TMC4) could act as a negative regulator of KCNQ1 (Kv7.1) potassium channel
TMC4 is a member of the transmembrane channel-like (TMC) protein family. In this family, TMC1 and TMC2 are thought to form the mechano-electrical transduction (MET) channel in the inner ear. On the other hand, the intrinsic functions of the other TMC family members (TMC3-8) are largely unknown. KCNQ1 (Kv7.1) channel is a voltage-gated potassium channel and plays crucial physiological roles with its auxiliary subunits, KCNE proteins (e.g. KCNQ1/KCNE1 complex contributes to cardiac repolarization, and KCNQ1/KCNE3 complex participates in epithelial ion transport). Recently, it was reported that TMC1 and TMC2 interacted with KCNQ1 and suppressed its K+ currents. However, the relationships between KCNQ1 and the other TMC proteins have not been examined. Here, we show a novel interaction and a functional association between overexpressed TMC4 and KCNQ1. The Bead Halo assay and FRET analysis revealed the physical interaction between these two proteins. Whole-cell patch clamp recording demonstrated that co-expression of TMC4 reduced KCNQ1 current densities without altering their voltage dependence and activation kinetics. This effect was also observed in the KCNQ1/KCNE1 and KCNQ1/KCNE3 channel complexes. A structural prediction using AlphaFold-Multimer suggested possible interaction sites between TMC4 and KCNQ1. Mutageneses, followed by patch clamp recording, suggested that specific amino acid residues at these sites contribute to the inhibitory effect of TMC4. These results indicate that TMC4 could function as a negative regulator of the KCNQ1 channel. Our findings could enhance the understanding of KCNQ1 channel regulation and propose potential research directions on the function of TMC4 under various physiological and pathological conditions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Biochimica et biophysica acta. Biomembranes
生物-生化与分子生物学
CiteScore
8.20
自引率
5.90%
发文量
175
审稿时长
2.3 months
期刊介绍:
BBA Biomembranes has its main focus on membrane structure, function and biomolecular organization, membrane proteins, receptors, channels and anchors, fluidity and composition, model membranes and liposomes, membrane surface studies and ligand interactions, transport studies, and membrane dynamics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


