揭示离子在水中“制造”和“破坏”结构的分子途径
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
阴离子在化学和生物过程中起着至关重要的作用。根据它们对电解质溶液粘度的影响,传统上它们被分类为“结构制造者”或“结构破坏者”。到目前为止,这种行为一直被认为是源于水的氢(H)键网络的单一重组机制,这可能与宏观性质一致。相关振动光谱(CVS)测量结果表明,情况并非如此。相反,阴离子通过多种不同的途径修饰水-水氢键,频率变化与电荷转移相关,强度变化量化了相互作用/方向交叉相关氢键数量的变化。阴离子影响水结构的不同方式可以用硬-软酸碱理论来解释。硬阴离子仅通过静电作用影响水的氢键。相比之下,软阴离子通过电荷转移削弱了氢键,但同时增加了它们的浓度。软阴离子的这两种效应在破坏/形成结构方面几乎相互抵消,导致其宏观行为与硬阴离子相似,尽管分子水平的效应截然不同。本文章由计算机程序翻译,如有差异,请以英文原文为准。
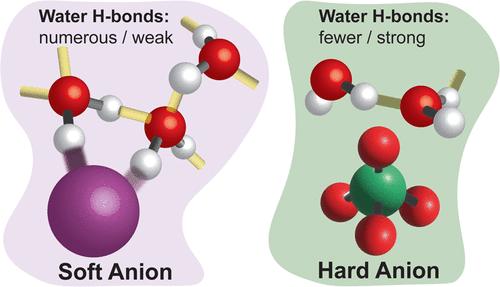
Unraveling the Molecular Pathways for Structure “Making” and “Breaking” by Ions in Water
Aqueous anions play a crucial role in chemical and biological processes. They are traditionally classified as “structure makers” or “structure breakers” based on their impact on the viscosity of electrolyte solutions. Until now, this behavior has been assumed to stem from a single restructuring mechanism of the hydrogen (H) bonding network of water, that could align with macroscopic properties. Correlated Vibrational Spectroscopy (CVS) measurements reveal that this is not the case. Rather, anions modify water–water H-bonds through multiple distinct pathways, with frequency shifts correlating with charge transfer, and intensity changes quantifying variations in the number of interacting/orientationally cross-correlated H-bonds. The different ways through which anions impact water structure can be explained in terms of Hard–Soft-Acid–Base theory. Hard anions only affect water H-bonds through electrostatics. By contrast, soft anions weaken the H-bonds via charge transfer but simultaneously increase their concentration. The two effects for soft anions nearly cancel each other out in terms of structure breaking/making, resulting in macroscopic behavior that is similar to hard anions in spite of dramatically different molecular-level effects.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


