抗糖尿病药物维格列汀n-亚硝胺类药物相关杂质n-亚硝基-维格列汀的合成、表征及LC-MS /MS灵敏方法的建立
IF 1.3
4区 化学
Q4 BIOCHEMICAL RESEARCH METHODS
引用次数: 0
摘要
n -亚硝基维格列汀杂质已被归类为遗传毒性。根据EMA,该杂质在维格列汀原料药中的含量必须控制在15ppm以内。这项工作证明了n -亚硝基-维格列汀杂质的合成和表征使用IR, NMR和LC-MS技术。以合成的n -亚硝基-维格列汀杂质为标准品,建立并验证了LC-MS /MS方法,检出限(LOD)为0.44 ppm,定量限(LOQ)为1.46 ppm。本文章由计算机程序翻译,如有差异,请以英文原文为准。
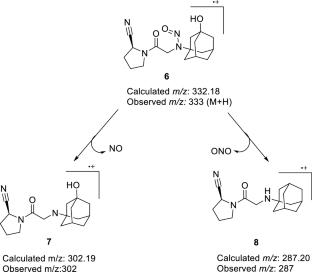
N-Nitroso-Vildagliptin, A New N-Nitrosamine Drug Substance Related Impurity (NDSRI) of Vildagliptin- An Antidiabetic Drug: Synthesis, Characterization and Development of Sensitive LC–MS/MS Method
The N-nitroso-vildagliptin impurity has been classified as genotoxic. According to the EMA, this impurity must be controlled within a limit of 15 ppm in the vildagliptin drug substance. This work demonstrates the synthesis and characterization of the N-nitroso-vildagliptin impurity using IR, NMR, and LC–MS techniques. The synthesized N-nitroso-vildagliptin impurity was used as a reference standard for the development and validation of an LC–MS/MS method, achieving a limit of detection (LOD) of 0.44 ppm and a limit of quantification (LOQ) of 1.46 ppm.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chromatographia
化学-分析化学
CiteScore
3.40
自引率
5.90%
发文量
103
审稿时长
2.2 months
期刊介绍:
Separation sciences, in all their various forms such as chromatography, field-flow fractionation, and electrophoresis, provide some of the most powerful techniques in analytical chemistry and are applied within a number of important application areas, including archaeology, biotechnology, clinical, environmental, food, medical, petroleum, pharmaceutical, polymer and biopolymer research. Beyond serving analytical purposes, separation techniques are also used for preparative and process-scale applications. The scope and power of separation sciences is significantly extended by combination with spectroscopic detection methods (e.g., laser-based approaches, nuclear-magnetic resonance, Raman, chemiluminescence) and particularly, mass spectrometry, to create hyphenated techniques. In addition to exciting new developments in chromatography, such as ultra high-pressure systems, multidimensional separations, and high-temperature approaches, there have also been great advances in hybrid methods combining chromatography and electro-based separations, especially on the micro- and nanoscale. Integrated biological procedures (e.g., enzymatic, immunological, receptor-based assays) can also be part of the overall analytical process.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


