卡拉胶-明胶配合物的分子结构和相互作用:计算与实验相结合的研究
IF 4.7
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
明胶是一种从胶原蛋白中提取的生物聚合物,由于其生物相容性和成胶性而广泛应用于生物医学和食品领域。然而,它的物理化学行为高度依赖于结构状态,是氨基酸组成、分子量分布和加工条件的函数。与多糖(如卡拉胶)形成的多电解质配合物可以显著改变明胶的构象稳定性和功能性能。在这项工作中,我们使用综合计算和实验方法在分子水平上研究了鱼明胶与硫酸酸化多糖卡拉胶(κ-, ι-和λ-型)之间的相互作用。采用分子对接和分子动力学模拟分析了卡拉胶的种类、长度和电荷对多糖-明胶配合物的结构和稳定性的影响,以及对酸性和中性ph下明胶分子的动力学和稳定性的影响。本文首次系统比较了三种类型的卡拉胶(k-,K -和λ-)在分子水平上,首次表明卡拉胶可以与不同化学计量的明胶分子(卡拉胶:明胶1:1、1:2和1:3)形成配合物。互补zeta电位分析证实了络合时ph依赖的电荷中和,支持了md预测的静电驱动缔合。扫描电镜显示复合凝胶中的微观结构重组是由于强烈的卡拉胶-明胶相互作用的结果。这些发现突出了分子结构和静电互补性在明胶-卡拉胶水凝胶中的关键作用,并有助于合理设计具有理想性能的明胶基凝胶体系。本文章由计算机程序翻译,如有差异,请以英文原文为准。
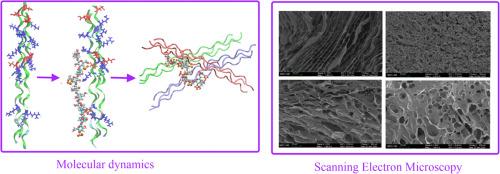
Molecular structure and interactions in carrageenan-gelatin complexes: A combined computational and experimental study
Gelatin, a biopolymer derived from collagen, is extensively employed in biomedical and food applications due to its biocompatibility and gel-forming properties. However, its physicochemical behavior is highly dependent on structural state, being a function of amino acid composition, molecular weight distribution, and processing conditions. The formation of polyelectrolyte complexes with polysaccharides, such as carrageenans, can significantly alter gelatin’s conformational stability and functional performance. In this work, we investigated the interactions between fish gelatin and sulfated polysaccharides carrageenans (κ-, ι-, and λ-types) at the molecular level using integrated computational and experimental approaches. Molecular docking and molecular dynamics simulations were applied to analyze the effect of carrageenan type, length, and charge on the structure and stability of polysaccharide-gelatin complexes, as well as on the dynamics and stability of gelatin molecule at acidic and neutral pH. This paper presents the first systematic comparison of the interaction of fish gelatin with all three types of carrageenans (k-, k- and λ-) at the molecular level and shows for the first time that carrageenans can form complexes with gelatin molecules of different stoichiometry (1:1, 1:2 and 1:3 carrageenan: gelatin). The complementary zeta potential analysis confirmed the pH-dependent charge neutralization upon complexation, supporting the MD-predicted electrostatic-driven association. Scanning electron microscopy demonstrated microstructural reorganization in composite gels as a result of strong carrageenan-gelatin interaction. These findings have highlighted the critical role of molecular structure and electrostatic complementarity in gelatin-carrageenan hydrogels and contribute to the rational design of gelatin-based gel systems with desired properties.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Molecular Structure
化学-物理化学
CiteScore
7.10
自引率
15.80%
发文量
2384
审稿时长
45 days
期刊介绍:
The Journal of Molecular Structure is dedicated to the publication of full-length articles and review papers, providing important new structural information on all types of chemical species including:
• Stable and unstable molecules in all types of environments (vapour, molecular beam, liquid, solution, liquid crystal, solid state, matrix-isolated, surface-absorbed etc.)
• Chemical intermediates
• Molecules in excited states
• Biological molecules
• Polymers.
The methods used may include any combination of spectroscopic and non-spectroscopic techniques, for example:
• Infrared spectroscopy (mid, far, near)
• Raman spectroscopy and non-linear Raman methods (CARS, etc.)
• Electronic absorption spectroscopy
• Optical rotatory dispersion and circular dichroism
• Fluorescence and phosphorescence techniques
• Electron spectroscopies (PES, XPS), EXAFS, etc.
• Microwave spectroscopy
• Electron diffraction
• NMR and ESR spectroscopies
• Mössbauer spectroscopy
• X-ray crystallography
• Charge Density Analyses
• Computational Studies (supplementing experimental methods)
We encourage publications combining theoretical and experimental approaches. The structural insights gained by the studies should be correlated with the properties, activity and/ or reactivity of the molecule under investigation and the relevance of this molecule and its implications should be discussed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


