具有抗神经炎潜能的紫杉叶c32 -环artane型三萜的1H nmr引导分离
IF 4.7
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
c32 -环artane型三萜由于其环丙烷环体系而表现出独特的1H NMR信号,使其与结构相关的类似物区别开来。本研究利用1H核磁共振引导分离技术,成功从紫杉叶中分离到7个新的典型环artane三萜,分别命名为Taxodium ascendens A-G(1-7),以及2个首次从植物中分离到的天然产物Taxodium H和I(8和9),以及2个已知的产物Taxodium ascendens 10和11。通过综合光谱技术,包括一维和二维核磁共振,严格阐明了这些未报道的分子的平面结构和绝对构型,并通过单晶x射线分析证实。生物活性评估显示,化合物7对BV-2小胶质细胞中lps诱导的一氧化氮(NO)产生具有有效的抑制作用,IC₅₀值为9.02 μM。进一步研究表明,化合物7可抑制IL-1β、TNF-α、COX-2、iNOS和IL-6 mRNA的表达,且呈剂量依赖性。后续研究进一步发现,化合物7能有效诱导BV-2细胞的炎性自噬。本文章由计算机程序翻译,如有差异,请以英文原文为准。
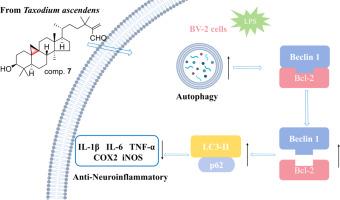
1H NMR-guided fractionation of C32-cycloartane type triterpenes from the leaves of Taxodium ascendens with anti-neuroinflammatory potential
C32-cycloartane type triterpenes exhibit distinctive 1H NMR signals attributable to their cyclopropane ring system, setting them apart from structurally related analogs. In this study, seven new typical cycloartane triterpenes, designated as taxcycloterpenes A-G (1–7), along with two natural products taxcycloterpenes H and I (8 and 9) isolated from plant for the first time, and two known ones (10 and 11), were successfully obtained from the leaves of Taxodium ascendens with the aid of 1H NMR-guided isolation. The planar structures and absolute configurations of these unreported identified molecules were rigorously elucidated through comprehensive spectroscopic techniques, including 1D and 2D NMR, and confirmed by single-crystal X-ray analysis. Bioactivity assessments revealed that compound 7 demonstrated a potent inhibitory effect on LPS-induced nitric oxide (NO) production in BV-2 microglial cells, with an IC₅₀ value of 9.02 μM. Further research indicated that compound 7 could inhibit the mRNA expression of IL-1β, TNF-α, COX-2, iNOS, and IL-6 in a dose-dependent manner. Subsequent investigations further revealed that compound 7 effectively induced the inflammatory autophagy in BV-2 cells.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Molecular Structure
化学-物理化学
CiteScore
7.10
自引率
15.80%
发文量
2384
审稿时长
45 days
期刊介绍:
The Journal of Molecular Structure is dedicated to the publication of full-length articles and review papers, providing important new structural information on all types of chemical species including:
• Stable and unstable molecules in all types of environments (vapour, molecular beam, liquid, solution, liquid crystal, solid state, matrix-isolated, surface-absorbed etc.)
• Chemical intermediates
• Molecules in excited states
• Biological molecules
• Polymers.
The methods used may include any combination of spectroscopic and non-spectroscopic techniques, for example:
• Infrared spectroscopy (mid, far, near)
• Raman spectroscopy and non-linear Raman methods (CARS, etc.)
• Electronic absorption spectroscopy
• Optical rotatory dispersion and circular dichroism
• Fluorescence and phosphorescence techniques
• Electron spectroscopies (PES, XPS), EXAFS, etc.
• Microwave spectroscopy
• Electron diffraction
• NMR and ESR spectroscopies
• Mössbauer spectroscopy
• X-ray crystallography
• Charge Density Analyses
• Computational Studies (supplementing experimental methods)
We encourage publications combining theoretical and experimental approaches. The structural insights gained by the studies should be correlated with the properties, activity and/ or reactivity of the molecule under investigation and the relevance of this molecule and its implications should be discussed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


