anderson型聚氧乙酸盐构建的过渡金属定向结构在碱性/海水介质中具有不同的电催化制氢性能
IF 4.7
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
本文采用水热法和溶剂热法合成了四种不同中心金属和不同结构的anderson型多金属氧酸盐基金属有机配合物(MOCs),分别为{Cu3(3- at)2[(TeMo6O24)](H2O)8}·6H2O(1)、{Co3(3- at)2[(TeMo6O24)](H2O)8}·6H2O(2)、{Ni3(3- at)2[(TeMo6O24)](H2O)8}·6H2O(3)、{Co2(3- at)2[CrMo6(OH)5O19](H2O)4}·7H2O(4)。采用单晶x射线衍射、红外光谱、粉末x射线衍射(PXRD)、x射线光电子能谱(XPS)和扫描电镜(SEM)对其组成和结构进行了表征。讨论了中心金属离子对结构的影响。系统研究了标题配合物1-4改性布碳电极(1-4 /CC)在碱性溶液和模拟海水条件下的电催化析氢性能。值得注意的是,当电流密度为10 mA cm-2时,1/CC在1 M KOH中表现出最低的过电位30.5 mV。此外,在模拟海水中,1/CC在10 mA cm-2下保持了34.8 mV的低过电位。中心金属及其配位模式对其电催化性能有显著影响。此外,配合物1对CO2 (CO2RR)的还原反应也表现出电催化活性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
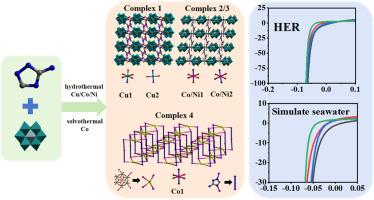
Transition metal-directed architectures constructed from Anderson-type polyoxmatelates with different electrocatalytic hydrogen production performance in alkaline/seawater medium
In this work, four new Anderson-type polyoxometalate-based metal-organic complexes (MOCs) with different central metals and various architectures, namely, {Cu3(3-AT)2[(TeMo6O24)](H2O)8}·6H2O (1), {Co3(3-AT)2[(TeMo6O24)](H2O)8}·6H2O (2), {Ni3(3-AT)2[(TeMo6O24)](H2O)8}·6H2O (3), {Co2(3-AT)2[CrMo6(OH)5O19](H2O)4}·7H2O (4), were synthesized via hydrothermal or solvothermal methods. Their composition and structure were characterized by single-crystal X-ray diffraction, IR spectroscopy, powder X-ray diffraction (PXRD), X-ray photoelectron spectroscopy (XPS) and scanning electron microscopy (SEM). The influence of the central metal ion on the structure was discussed. The electrocatalytic hydrogen evolution (HER) performances of the title complexes 1–4 modified cloth carbon electrodes (1–4/CC) in alkaline solution and simulated seawater condition were systematically studied. Notably, 1/CC exhibited the lowest overpotential of 30.5 mV at the current density of 10 mA cm-2 for HER in 1 M KOH. Furthermore, in simulated seawater, 1/CC maintained a low overpotential of 34.8 mV at 10 mA cm-2 for HER. The central metals and their coordination modes show a significant influence on their electrocatalytic performance. In addition, complex 1 also shows electrocatalytic activity towards the reduction reaction of the CO2 (CO2RR).
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Molecular Structure
化学-物理化学
CiteScore
7.10
自引率
15.80%
发文量
2384
审稿时长
45 days
期刊介绍:
The Journal of Molecular Structure is dedicated to the publication of full-length articles and review papers, providing important new structural information on all types of chemical species including:
• Stable and unstable molecules in all types of environments (vapour, molecular beam, liquid, solution, liquid crystal, solid state, matrix-isolated, surface-absorbed etc.)
• Chemical intermediates
• Molecules in excited states
• Biological molecules
• Polymers.
The methods used may include any combination of spectroscopic and non-spectroscopic techniques, for example:
• Infrared spectroscopy (mid, far, near)
• Raman spectroscopy and non-linear Raman methods (CARS, etc.)
• Electronic absorption spectroscopy
• Optical rotatory dispersion and circular dichroism
• Fluorescence and phosphorescence techniques
• Electron spectroscopies (PES, XPS), EXAFS, etc.
• Microwave spectroscopy
• Electron diffraction
• NMR and ESR spectroscopies
• Mössbauer spectroscopy
• X-ray crystallography
• Charge Density Analyses
• Computational Studies (supplementing experimental methods)
We encourage publications combining theoretical and experimental approaches. The structural insights gained by the studies should be correlated with the properties, activity and/ or reactivity of the molecule under investigation and the relevance of this molecule and its implications should be discussed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


