探索密度泛函理论,深入了解从4-(二乙胺)水杨醛衍生的二羟基希夫碱的烯丙亚胺-酮胺互变异构性,并研究其作为抗糖尿病和抗氧化剂的用途
IF 4.7
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
通过4-(二乙胺)水杨醛与氨基酚衍生物的缩合反应,制备了5-氯-2-((4-(二乙胺)-2-羟基苄基)氨基)苯酚(DAC)、2-((4-(二乙胺)-2-羟基苄基)氨基)-4-甲基苯酚(DAM)和5-(二乙胺)-2-((2-羟基-4-硝基)亚胺)甲基苯酚(DAN) 3个二羟基席夫碱。用光谱和元素分析技术对化合物进行了鉴定。将DAC和DAM(烯胺型)的晶体结构在甲醇中于60 -70℃生长,形成酮胺型,记为DAC ‘和DAM ’。DAC ‘和DAM ’的分子构象表明,4-二乙胺和氨基苯基环与亚甲基官能团共面,C13-C8-C7-N1的二面角分别为-178.78º(DAC ‘)和-174.99º(DAM ’), C1/ C3-C6 / C4-N1-C7的二面角分别为179.16º(DAC ‘)和174.08º(DAM ’)。DAC ‘和DAM ’的C7-N1键长较长,c9 - 01键长稍短,证实了它们与DAC和DAM相关的互变异构性质。我们使用密度泛函理论(DFT)来研究DAM、DAC和DAN的结构、电子和互变异构性质。值得注意的是,所有化合物都表现出单一的分子内氢键,这稳定了其结构并促进了质子转移以产生酮胺互变异构体。然而,由于双分子间氢键的存在,烯胺互变异构体更加稳定,因此支持DAC ‘和DAM ’在DAC和DAM之上的结晶。化合物DAN的能带隙最小,为6.158 eV,是三种化合物中最具活性和极化性的分子。通过铁还原能力(FRAP)、2,2-二苯基-1-吡啶酰肼(DPPH)和一氧化氮测定来评价化合物的抗氧化能力。DAN的IC50值为0.51 μΜ,对一氧化氮自由基的清除能力优于槲皮素的IC50值为149.96 μΜ。采用α-葡萄糖苷酶和α-淀粉酶抑制试验评价其抗糖尿病潜能。对于α-淀粉酶实验,IC50值为0.13 μΜ的DAC与IC50值为0.11 μΜ的阿卡波糖表现出几乎相等的能力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
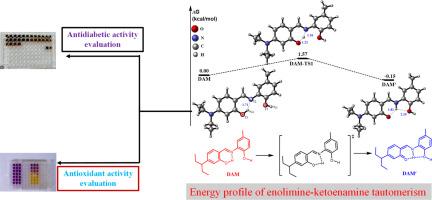
Exploring density functional theory to gain insight into the enolimine-ketoenamine tautomerism of dihydroxyl Schiff bases derived from 4-(diethylamino)salicylaldehyde and investigating their use as antidiabetes and antioxidant agents
Three dihydroxyl Schiff bases namely, 5-chloro-2-((4-(diethylamino)-2-hydroxybenzylidene)amino)phenol (DAC), 2-((4-(diethylamino)-2-hydroxybenzylidene)amino)-4-methylphenol (DAM) and 5-(diethylamino)-2-(((2-hydroxy-4-nitrophenyl)imino)methyl)phenol (DAN) were prepared by the condensation reaction between 4-(diethylamino)salicylaldehyde and amino-phenol derivatives. The compounds were elucidated using spectroscopic and elemental analysis techniques. Attempts to grow the crystal structure of DAC and DAM (enolimine form) at 60 –70 °C in methanol resulted in the formation of their ketoenamine form and are denoted as DAC′ and DAM′. The molecular conformation of DAC′ and DAM′ revealed that the 4-diethylamino and the aminophenol phenyl rings are coplanar with the azomethine functional group as evident by C13—C8—C7—N1 dihedral angles of -178.78º (DAC′) and -174.99º (DAM′) as well as C1/C3—C6/C4—N1—C7 dihedral angles of 179.16º (DAC′) and 174.08º (DAM′). The longer bond length observed in C7—N1 as well as slightly shorter bond length observed in C9—O1 of DAC′ and DAM′ affirms their tautomeric properties related to DAC and DAM. We used Density functional theory (DFT) to investigate the structural, electronic, and tautomeric properties of DAM, DAC, and DAN. Notably, all the compounds exhibit single intramolecular hydrogen bonding that stabilizes its structure and facilitates proton transfer to yield its ketoenamine tautomer. However, the enamine tautomer is more stabilized due to the presence of dual intermolecular hydrogen bonding, hence supporting the crystallization of DAC′ and DAM′ over DAC and DAM. Compound DAN with the lowest energy bandgap of 6.158 eV is suggested to be the most reactive and polarizable molecule among the three compounds. Ferric reducing ability power (FRAP), 2,2-diphenyl-1-picrylhydrazyl (DPPH) as well as nitric oxide assays were explored to evaluate the antioxidant potential of the compounds. They exhibited good to moderate antioxidant activities i.e., DAN with IC50 value of 0.51 μΜ scavenge nitric oxide radical better than quercetin with an IC50 value of 149.96 μΜ. The evaluation of the antidiabetic potential was carried out using α-glucosidase and α-amylase inhibition assays. For the α-amylase assay, DAC with an IC50 value of 0.13 μΜ exhibited almost equivalent capacity as acarbose with IC50 value of 0.11 μΜ.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Molecular Structure
化学-物理化学
CiteScore
7.10
自引率
15.80%
发文量
2384
审稿时长
45 days
期刊介绍:
The Journal of Molecular Structure is dedicated to the publication of full-length articles and review papers, providing important new structural information on all types of chemical species including:
• Stable and unstable molecules in all types of environments (vapour, molecular beam, liquid, solution, liquid crystal, solid state, matrix-isolated, surface-absorbed etc.)
• Chemical intermediates
• Molecules in excited states
• Biological molecules
• Polymers.
The methods used may include any combination of spectroscopic and non-spectroscopic techniques, for example:
• Infrared spectroscopy (mid, far, near)
• Raman spectroscopy and non-linear Raman methods (CARS, etc.)
• Electronic absorption spectroscopy
• Optical rotatory dispersion and circular dichroism
• Fluorescence and phosphorescence techniques
• Electron spectroscopies (PES, XPS), EXAFS, etc.
• Microwave spectroscopy
• Electron diffraction
• NMR and ESR spectroscopies
• Mössbauer spectroscopy
• X-ray crystallography
• Charge Density Analyses
• Computational Studies (supplementing experimental methods)
We encourage publications combining theoretical and experimental approaches. The structural insights gained by the studies should be correlated with the properties, activity and/ or reactivity of the molecule under investigation and the relevance of this molecule and its implications should be discussed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


