含β-二酮酸酯的钌(II)-芳烃配合物:通过实验数据、对接和DFT计算相结合评价利什曼尼杀虫活性
IF 4.7
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
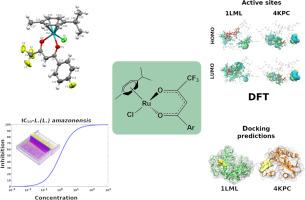
本研究考察了四种β-二酮、4,4,4-三氟-1-苯基-1,3-丁二酮(HL1)、2-烯基三氟丙酮(HL2)、4,4,4-三氟-1-(4-溴苯基)-1,3-丁二酮(HL3)和4,4,4-三氟-1-(4-氟苯基)-1,3-丁二酮(HL4)及其对应的钌(II)芳烃配合物(通式[Ru(ƞ6-p-cymene)(L1-4)Cl](1-4))的杀毒活性。通过FTIR, UV-Vis, 1H和19F{1H} NMR以及元素分析对化合物进行了全面表征,确定了新配合物4的晶体结构,证实了预期的钢琴凳几何形状。β-二酮类化合物对亚马河利什曼原虫具有良好~中等的抗利什曼原虫活性,其中以HL4活性最强(IC50 = 9.53 μM)。相反,与Ru(II)配位通常会降低生物活性,配合物1-4仅表现出中等至低的影响(IC50 = 55.8 - 90.5 μM)。RAW 264.7巨噬细胞的细胞毒性实验显示,对配体和复合物的选择性有限(SI≤2.0)。利用TD-DFT进行的电子结构分析证实了实验紫外-可见光谱的主要特征,确定了配体内电荷转移(ILCT)、配体到配体电荷转移(LLCT)和金属到配体电荷转移(MCLT)转变。前沿轨道分析表明,HOMO主要集中在Ru(II)及其配位球上,而LUMO则离域在β-二酮酸配体上,与MLCT跃迁一致。最后,在复合物4上进行的分子对接和量子力学计算显示了与两个相关利什曼原虫蛋白靶点(1LML和4KPC)的良好结合亲和力,并得到了结合位点内电荷转移相互作用的支持。总之,虽然Ru(II)配合物在体外表现出有限的利什曼尼毒力,但这项工作为影响活性的结构和电子因素提供了有价值的见解。这些结果突出了合理修饰β-二酮酸配体和Ru(II)配位环境作为优化未来抗利什曼候选物的策略的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Ruthenium(II)-arene complexes containing β-diketonates: Leishmanicidal activity evaluated by the combination of experimental data, docking and DFT calculations
In this study, we investigated the leishmanicidal activity of four β-diketones, 4,4,4-trifluoro-1-phenyl-1,3-butanedione (HL1), 2-thenoyltrifluoroacetone (HL2), 4,4,4-trifluoro-1-(4-bromophenyl)-1,3-butanedione (HL3) and 4,4,4-trifluoro-1-(4-fluorophenyl)-1,3-butanedione (HL4), and their corresponding ruthenium(II) arene complexes with general formula [Ru(ƞ6-p-cymene)(L1–4)Cl] (1–4). The compounds were fully characterized by FTIR, UV–Vis, 1H and 19F{1H} NMR, and elemental analysis, and the crystal structure of the new complex 4 was determined, confirming the expected piano-stool geometry. The β-diketones exhibited good to moderate antileishmanial activity against Leishmania (L.) amazonensis promastigotes, with HL4 being the most active (IC50 = 9.53 μM). In contrast, coordination to Ru(II) generally reduced biological activity, with complexes 1–4 showing only moderate to low effects (IC50 = 55.8 – 90.5 μM). Cytotoxicity assays on RAW 264.7 macrophages revealed limited selectivity for both ligands and complexes (SI ≤ 2.0). Electronic structure analyses using TD-DFT confirmed the main features of the experimental UV–Vis spectra, identifying intraligand charge transfer (ILCT), ligand-to-ligand charge transfer (LLCT), and metal-to-ligand charge transfer (MCLT) transitions. Frontier orbital analysis indicated that the HOMO is mainly centered on Ru(II) and its coordination sphere, while the LUMO is delocalized on the β-diketonate ligands, consistent with MLCT transitions. Finally, molecular docking and quantum mechanical calculations performed on complex 4 revealed promising binding affinities toward two relevant Leishmania protein targets (1LML and 4KPC), supported by charge transfer interactions within this binding sites. Altogether, while the Ru(II) complexes demonstrated limited in vitro leishmanicidal potency, this work provides valuable insights into the structural and electronic factors influencing activity. These results highlight the potential of rationally modifying β-diketonate ligands and the Ru(II) coordination environment as a strategy to optimize future antileishmanial candidates.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Molecular Structure
化学-物理化学
CiteScore
7.10
自引率
15.80%
发文量
2384
审稿时长
45 days
期刊介绍:
The Journal of Molecular Structure is dedicated to the publication of full-length articles and review papers, providing important new structural information on all types of chemical species including:
• Stable and unstable molecules in all types of environments (vapour, molecular beam, liquid, solution, liquid crystal, solid state, matrix-isolated, surface-absorbed etc.)
• Chemical intermediates
• Molecules in excited states
• Biological molecules
• Polymers.
The methods used may include any combination of spectroscopic and non-spectroscopic techniques, for example:
• Infrared spectroscopy (mid, far, near)
• Raman spectroscopy and non-linear Raman methods (CARS, etc.)
• Electronic absorption spectroscopy
• Optical rotatory dispersion and circular dichroism
• Fluorescence and phosphorescence techniques
• Electron spectroscopies (PES, XPS), EXAFS, etc.
• Microwave spectroscopy
• Electron diffraction
• NMR and ESR spectroscopies
• Mössbauer spectroscopy
• X-ray crystallography
• Charge Density Analyses
• Computational Studies (supplementing experimental methods)
We encourage publications combining theoretical and experimental approaches. The structural insights gained by the studies should be correlated with the properties, activity and/ or reactivity of the molecule under investigation and the relevance of this molecule and its implications should be discussed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


