纳米结构CuZn双金属材料电化学检测人工唾液中肌酐
IF 4.7
3区 材料科学
Q2 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
在本研究中,将Zn作为保护和牺牲共金属与电活性金属Cu结合,形成电沉积CuZn材料用于肌酐检测。以乙二胺-四乙酸(EDTA)为添加剂,制备了四种铜锌比(1:2、1:4、1:8和1:12)。理化性质表明,在1:2和1:12的比例下,锌原子竞争率分别为43%和30%,而在1:4和1:8的比例下,锌含量分别为13%和3%。电化学评价表明,在较低锌浓度下,肌酐检测性能(活性和重复性)有所提高,以1:4 CuZn组成(87% Cu, 13% Zn)为最佳。在含20 mM NaCl和2 mM KCl的0.1 M磷酸盐缓冲溶液(PBS)中,该优化组合对0 ~ 500 μM范围内的肌酐检测具有良好的线性响应,相关系数(R2)为0.99。在PBS中1:5稀释的人工唾液中保持线性(R2 = 0.98),但重复性降低。为了解决这个问题,使用丝网印刷电极(spe)建立了一个微环境,提高了重复性,同时在0-200 μM范围内保持了线性(R2 = 0.98)。这些发现强调,当锌作为共金属时,低稀释率(1:5)可以有效地用于肌酐检测。锌不仅可以保护Cu,还可以促进Cu+的形成,支持Cu+ -和Cu2+ -肌酸酐配合物的双重检测机制。本文章由计算机程序翻译,如有差异,请以英文原文为准。
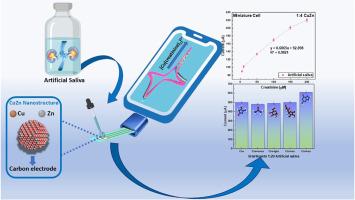
Electrochemical detection of creatinine in artificial saliva using nanostructured CuZn bimetallic materials
In this study, Zn was incorporated as a protective and sacrificial co-metal alongside Cu, the electroactive metal, to form electrodeposited CuZn materials for creatinine detection. Four Cu-to-Zn ratios (1:2, 1:4, 1:8 and 1:12) were prepared using ethylenediamine-tetraacetic acid (EDTA) as an additive. Physicochemical characterization revealed Zn atomic contests of 43 %, and 30 % for the 1:2 and 1:12 ratios, respectively, while the 1:4 and 1:8 ratios yielded lower Zn contents of 13 % and 3 %. Electrochemical evaluation demonstrated improved creatinine detection performance (in activity and repeatability) at the lower Zn concentrations, with the 1:4 CuZn composition (87 % Cu, 13 % Zn) showing the best results. This optimal composition exhibited a linear response for creatinine detection in the range of 0–500 μM in a 0.1 M phosphate buffer solution (PBS) containing 20 mM NaCl and 2 mM KCl, with a correlation coefficient (R2) of 0.99. Linearity was maintained in artificial saliva diluted 1:5 in PBS (R2 = 0.98), although repeatability was reduced. To address this, a micro-environment was established using screen-printed electrodes (SPEs), enhancing repeatability while preserving linearity (R2 = 0.98) within a 0–200 μM range. These findings highlight that low dilution rates (1:5) can be effectively used for creatinine detection when Zn is employed as a co-metal. Zn not only protects Cu but also facilitates the formation of Cu+ species, supporting a dual detection mechanism involving both Cu+– and Cu2+–creatinine complexes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Materials Chemistry and Physics
工程技术-材料科学:综合
CiteScore
8.70
自引率
4.30%
发文量
1515
审稿时长
69 days
期刊介绍:
Materials Chemistry and Physics is devoted to short communications, full-length research papers and feature articles on interrelationships among structure, properties, processing and performance of materials. The Editors welcome manuscripts on thin films, surface and interface science, materials degradation and reliability, metallurgy, semiconductors and optoelectronic materials, fine ceramics, magnetics, superconductors, specialty polymers, nano-materials and composite materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


