基于机器学习的单频粘弹性脑白质预测-一个数据科学框架
IF 6.3
2区 医学
Q1 BIOLOGY
引用次数: 0
摘要
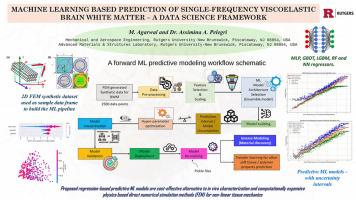
利用活体磁共振弹性成像(MRE)和弥散张量成像(DTI)表征脑白质(BWM)是一个昂贵且耗时的过程。数值模拟方法,如有限元模型(fem),也面临着保真度、计算资源和准确捕捉脑组织复杂生物物理行为的限制。为了解决实验数据缺乏的问题,研究人员正在探索机器学习(ML)作为预测脑组织机械特性的替代方法。本文提出了一种机器学习工作流,用于使用fem衍生数据预测BWM的均匀粘弹性特性。合成FE数据集来源于敏感性分析,其中使用由轴突,髓磷脂和胶质基质组成的三相二维复合模型来模拟谐波剪切应力下的横向力学行为。该数据集用于训练和验证旨在预测频率相关机械响应的机器学习模型。所提出的ML管道结合了微观结构特征,如纤维体积分数、本征相位模量和轴突几何形状,以构建和训练回归模型。采用特征选择和超参数优化来提高预测精度。基于决策树的模型优于其他方法,而SHAP解释显示神经胶质模量和纤维体积分数显著影响预测。该框架为体内表征和计算昂贵的物理直接数值模拟方法(FEM)提供了一种具有成本效益的替代方案。它还将为未来的机器学习驱动的逆模型提供基础,以探索各种脑物质成分对神经成像特征的影响,潜在地为衰老、痴呆和创伤性脑损伤的研究提供信息。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Machine learning based prediction of single-frequency viscoelastic brain white matter – A data science framework
Characterizing brain white matter (BWM) using in vivo Magnetic Resonance Elastography (MRE) and Diffusion Tensor Imaging (DTI) is a costly, time-intensive process. Numerical modeling approaches, such as finite element models (FEMs), also face limitations in fidelity, computational resources, and accurately capturing the complex bio-physical behavior of brain tissues. To address the scarcity of experimental data, researchers are exploring machine learning (ML) as a surrogate for predicting the mechanical properties of brain tissues. Here in, an ML workflow is proposed for predicting the homogenized viscoelastic properties of BWM using FEM-derived data. The synthetic FE dataset originates from a sensitivity analysis, whereby a triphasic 2D composite model, consisting of axons, myelin, and glial matrix, was used to simulate transverse mechanical behavior under harmonic shear stress. This dataset is utilized to train and validate machine learning models aimed at predicting the frequency-dependent mechanical response.
The proposed ML pipeline incorporates microstructural features such as fiber volume fraction, intrinsic phase moduli, and axonal geometry to build and train regression models. Feature selection and hyperparameter optimization were applied to improve prediction accuracy. Decision tree-based models outperformed other approaches, while SHAP interpretation revealed that glial moduli and fiber volume fraction significantly influenced the predictions. This framework offers a cost-effective alternative to in vivo characterization and computationally expensive physics based direct numerical simulation methods (FEM). It would also provide a basis for future ML-driven inverse models to explore the impact of various brain matter constituents on neuroimaging characteristics, potentially informing studies on aging, dementia, and traumatic brain injuries.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Computers in biology and medicine
工程技术-工程:生物医学
CiteScore
11.70
自引率
10.40%
发文量
1086
审稿时长
74 days
期刊介绍:
Computers in Biology and Medicine is an international forum for sharing groundbreaking advancements in the use of computers in bioscience and medicine. This journal serves as a medium for communicating essential research, instruction, ideas, and information regarding the rapidly evolving field of computer applications in these domains. By encouraging the exchange of knowledge, we aim to facilitate progress and innovation in the utilization of computers in biology and medicine.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


