一氯化碘与吡嗪分子和离子配合物的结构和键合
IF 3.2
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
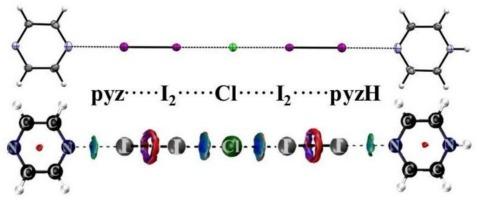
通过x射线衍射分析首次确定了ICl与吡嗪(pyz)的分子和离子化合物的结构。在分子复合物ICl∙pyz∙ICl(1)中,吡嗪作为双齿非螯合配体。离子配合物[Hpyz]+[ICl2]−(2)和[I2∙pyz…Hpyz∙I2]Cl(3)表现出明显的配位键模式。2的结构基序具有无限的[…pyz…H-pyz…]+氢键阳离子链和不同的[ICl2] -反离子,它们不仅通过静电阴离子-阳离子吸引与阳离子链相互作用,还通过弱CH⋯Cl氢键相互作用。在非常特殊的结构3中,碘分子充当双齿刘易斯酸,桥接氢键阳离子[pyz…Hpyz]+和氯阴离子,形成无限的几乎线性链(…I2…pyz…Hpyz+…I2…Cl−…I2∙pyz…Hpyz+…I2…Cl−…)∞。量子化学计算可以使反应性合理化,并在化合物中建立化学键。主要结合基序由[pyz…Hpyz]+片段中的氢键和供体-受体卤素键Cl−→I-I←N控制,其中碘分子作为双齿刘易斯酸。两种Lewis酸I2∙pyz和I2∙Hpyz+在3中的Cl−阴离子周围的异常线性排列是由于外围吡嗪环之间π-π桩的填充效应。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Structure and bonding in molecular and ionic complexes of iodine monochloride with pyrazine
Structures of molecular and ionic compounds of ICl with pyrazine (pyz) have been established for the first time by X-ray diffraction structural analysis. In the molecular complex ICl∙pyz∙ICl (1) pyrazine serves as bidentate nonchelating ligand. The ionic complexes [Hpyz]+[ICl2]− (2) and [I2∙pyz…Hpyz∙I2]Cl (3) exhibit distinct coordination bonding patterns. The structural motif of 2 features infinite […pyz…H-pyz…]+ hydrogen bonded cationic chains and distinct [ICl2]− counterions, which interact with cationic chain not only through electrostatic anion–cation attraction but also via weak C![]() H⋯Cl hydrogen bonds.
H⋯Cl hydrogen bonds.
In very peculiar structure of 3, the iodine molecules act as bidentate Lewis acid, bridging the hydrogen-bonded cations [pyz…Hpyz]+ and the chloride anions in the infinite, almost linear chains (…I2…pyz…Hpyz+…I2…Cl−…I2∙pyz…Hpyz+…I2…Cl−…)∞. Quantum chemical computations allowed to rationalize reactivity and establish a chemical bonding in the compounds. The dominant binding motif is governed by hydrogen bonds in [pyz…Hpyz]+ fragments and donor-acceptor halogen bonds Cl− → I–I ← N, where iodine molecules serve as bidentate Lewis acids. Unusual linear arrangement of two Lewis acids I2∙pyz and I2∙Hpyz+ around Cl− anion in 3 is facilitated due to packing effects forced by π-π staking between peripheral pyrazine rings.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Inorganica Chimica Acta
化学-无机化学与核化学
CiteScore
6.00
自引率
3.60%
发文量
440
审稿时长
35 days
期刊介绍:
Inorganica Chimica Acta is an established international forum for all aspects of advanced Inorganic Chemistry. Original papers of high scientific level and interest are published in the form of Articles and Reviews.
Topics covered include:
• chemistry of the main group elements and the d- and f-block metals, including the synthesis, characterization and reactivity of coordination, organometallic, biomimetic, supramolecular coordination compounds, including associated computational studies;
• synthesis, physico-chemical properties, applications of molecule-based nano-scaled clusters and nanomaterials designed using the principles of coordination chemistry, as well as coordination polymers (CPs), metal-organic frameworks (MOFs), metal-organic polyhedra (MPOs);
• reaction mechanisms and physico-chemical investigations computational studies of metalloenzymes and their models;
• applications of inorganic compounds, metallodrugs and molecule-based materials.
Papers composed primarily of structural reports will typically not be considered for publication.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


