巯基氨基脲配体二μ-乙酰氨基双核Zn(II)配合物的合成、表征及体外抗癌活性研究
IF 3.2
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
本文报道了二μ-乙酰氨基桥接Zn(II)配合物{[Zn(μ-ac)CyHCT]2和[Zn(μ-ac)CyBHCT]2}含有硫代氨基脲偶联肼-1-碳硫酰胺配体{n-环己基-2-(1-(吡啶-2-基)乙基)肼-1-碳硫酰胺(HCyHCT)和n-环己基-2-(苯基(吡啶-2-基)亚甲基)肼-1-碳硫酰胺(HCyBHCT)}。配合物的晶体结构表明,锌(II)中心由三个硫代氨基脲原子(两个N和一个S)和来自两个乙酸基的一个O原子配位成扭曲的三角双锥体构型。此外,[Zn(μ-ac)CyBHCT]2中的二μ-乙酰氨基桥呈连锁异构。进一步研究了该配体和复合物对HT-29(人结肠癌细胞)和DL(道尔顿淋巴瘤细胞)癌细胞的抗癌活性。结果表明,这些化合物对HT-29细胞具有较强的细胞毒活性。其中,[Zn(μ-ac)CyHCT]2在24 h和48 h时的IC50值分别接近150 μM和110 μM,具有最显著的细胞毒性反应。我们还通过DAPI染色、AO/EtBr双染色、ROS生成和线粒体膜电位测量等进一步研究了细胞死亡的机制,发现ROS生成的减少和增加,强调了线粒体依赖性凋亡是肿瘤细胞死亡的主要机制。此外,对HEK-293(正常肾)细胞进行了进一步的生物相容性测试,表明该复合物具有生物相容性,对正常细胞具有边际细胞毒性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
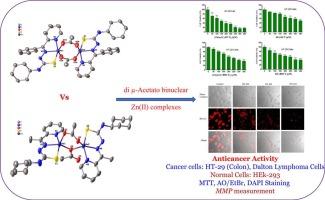
Synthesis, characterizations, and in vitro anticancer activity of di μ-acetato binuclear Zn(II) complexes with thiosemicarbazone ligands
Here, we report di μ-acetato bridged Zn(II) complexes {[Zn(μ-ac)CyHCT]2 and [Zn(μ-ac)CyBHCT]2} containing thiosemicarbazone alias hydrazine-1-carbothioamide ligands {N-Cyclohexyl-2-(1-(pyridin-2-yl)ethylidene)hydrazine-1-carbothioamide (HCyHCT) and N-Cyclohexyl-2-(phenyl(pyridin-2-yl)methylene)hydrazine-1-carbothioamide (HCyBHCT)}. The crystal structure of complexes shows that the Zn(II) centre is coordinated in a distorted trigonal bipyramidal configuration by three thiosemicarbazone atoms (two N and one S) and by an O atom from each of the two acetate groups. Further, di μ-acetato bridges in [Zn(μ-ac)CyBHCT]2 are showing linkage isomerism. The ligand and complexes were further studied for their anticancer activities against the HT-29 (human colon) and DL (Dalton's lymphoma cells) cancer cells. The results imply that these compounds have superior cytotoxic activity against HT-29 cells. Additionally, among all the compounds [Zn(μ-ac)CyHCT]2 has the most significant cytotoxic response with an IC50 value close to 150 μM and 110 μM upon 24 h and 48 h incubation, respectively. Further assays DAPI staining, AO/EtBr dual staining, ROS production, and mitochondrial membrane potential measurement, were also performed to gain insights into the mechanism of cell death, and found that reduced and increased ROS production, highlighting mitochondrial-dependent apoptosis as the major mechanism for tumour cell death. In addition, the biocompatibility was further tested with HEK-293 (normal kidney) cells, which suggests the complex is biocompatible with a marginal cytotoxicity to normal cells.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Inorganica Chimica Acta
化学-无机化学与核化学
CiteScore
6.00
自引率
3.60%
发文量
440
审稿时长
35 days
期刊介绍:
Inorganica Chimica Acta is an established international forum for all aspects of advanced Inorganic Chemistry. Original papers of high scientific level and interest are published in the form of Articles and Reviews.
Topics covered include:
• chemistry of the main group elements and the d- and f-block metals, including the synthesis, characterization and reactivity of coordination, organometallic, biomimetic, supramolecular coordination compounds, including associated computational studies;
• synthesis, physico-chemical properties, applications of molecule-based nano-scaled clusters and nanomaterials designed using the principles of coordination chemistry, as well as coordination polymers (CPs), metal-organic frameworks (MOFs), metal-organic polyhedra (MPOs);
• reaction mechanisms and physico-chemical investigations computational studies of metalloenzymes and their models;
• applications of inorganic compounds, metallodrugs and molecule-based materials.
Papers composed primarily of structural reports will typically not be considered for publication.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


