葡萄糖酸盐存在下Eu(III)在C-S-H相上的吸附:分子动力学研究
IF 3.4
3区 地球科学
Q1 GEOCHEMISTRY & GEOPHYSICS
引用次数: 0
摘要
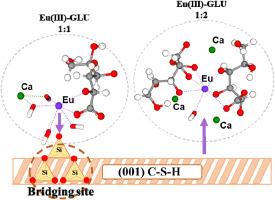
水泥是放射性废物储存库中的关键屏障材料,其中硅酸盐水合钙(C-S-H)相在固定阳离子放射性核素方面起着核心作用。然而,来自添加剂或废物的有机配体可以通过形成可溶性配合物和竞争表面吸附来增强放射性核素的迁移性。在这项研究中,开发了一个表面模型,将实验观察与C-S-H结构的理论见解相结合,从而能够对最可能的吸附位点进行详细采样。通过分子动力学(MD)模拟和平均力势(PMF)计算,研究人员了解了有机添加剂如何影响胶凝材料中三价锕系元素和镧系元素的吸附和迁移。Eu(III)被认为是核废料中可能存在的关键三价放射性核素的模型,即Pu(III)和Am(III),基于它们相似的电荷尺寸(z/d)比,葡萄糖酸盐被选为模型有机配体。Eu(III)/ C-S-H二元体系的结果证实了其强吸附作用,并表明最常见的吸附位点是表面去质子化的硅醇基团。二元系统的结果与Eu(III)和Cm(III)的时间分辨激光荧光光谱数据一致。根据葡萄糖酸盐浓度的不同,C-S-H相对Eu(III)吸收的两个主要影响已经被发现:(a)低配体浓度下1:1 Eu(III)-GLU复合物的吸收;(b)形成稳定的三元Ca-Eu (III)-GLU水溶液配合物,在高配体浓度下不吸附。考虑三元配合物C-S-H /Eu(III)/GLU的形成对于整体解释和理解该体系是重要的。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Sorption of Eu(III) on C–S–H phases in the presence of gluconate: A molecular dynamics study
Cement is a key barrier material in radioactive waste repositories, where calcium-silicate-hydrate (C–S–H) phases play a central role in immobilizing cationic radionuclides. However, organic ligands, originating from additives or waste, can enhance radionuclide mobility by forming soluble complexes and competing for surface sorption. In this study, a surface model was developed that combines experimental observations with theoretical insights into C–S–H structure, enabling detailed sampling of the most probable sorption sites. Molecular dynamics (MD) simulations and potential of mean force (PMF) calculations were used to develop molecular-scale understanding of how organic additives influence the adsorption and mobility of trivalent actinides and lanthanides in cementitious materials. Eu(III) was considered as a model of key trivalent radionuclides expected in nuclear waste, i.e. Pu(III) and Am(III), based on their similar charge-to-size (z/d) ratios, and gluconate was chosen as a model organic ligand. The results from the Eu(III)/C–S–H binary system confirmed strong sorption and showed that the most common sorption sites are the deprotonated silanol groups of the surface. Results obtained for the binary system are in line with Time Resolved Laser Fluorescence Spectroscopy data available for Eu(III) and Cm(III). Depending on gluconate concentration, two main effects on Eu(III) uptake on the C–S–H phases have been found: (a) sorption of the 1:1 Eu(III)-GLU complex at low ligand concentration; (b) formation of a stable ternary Ca–Eu(III)-GLU aqueous complex that does not sorb at high ligand concentration. It is important to consider formation of the ternary complex C–S–H/Eu(III)/GLU for the overall interpretation and understanding of the system.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Applied Geochemistry
地学-地球化学与地球物理
CiteScore
6.10
自引率
8.80%
发文量
272
审稿时长
65 days
期刊介绍:
Applied Geochemistry is an international journal devoted to publication of original research papers, rapid research communications and selected review papers in geochemistry and urban geochemistry which have some practical application to an aspect of human endeavour, such as the preservation of the environment, health, waste disposal and the search for resources. Papers on applications of inorganic, organic and isotope geochemistry and geochemical processes are therefore welcome provided they meet the main criterion. Spatial and temporal monitoring case studies are only of interest to our international readership if they present new ideas of broad application.
Topics covered include: (1) Environmental geochemistry (including natural and anthropogenic aspects, and protection and remediation strategies); (2) Hydrogeochemistry (surface and groundwater); (3) Medical (urban) geochemistry; (4) The search for energy resources (in particular unconventional oil and gas or emerging metal resources); (5) Energy exploitation (in particular geothermal energy and CCS); (6) Upgrading of energy and mineral resources where there is a direct geochemical application; and (7) Waste disposal, including nuclear waste disposal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


