金属氯化物改性深共晶溶剂萃取脱硫模型喷气燃料
IF 5.2
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
原油中含有由杂环烃和石蜡烃组成的各种有机硫化合物。在原油的升级过程中,这些含硫化合物不可避免地最终出现在最终产品中,包括不同的燃料馏分(汽油、煤油、柴油等)。喷气燃料就是这样一种原油馏分。高含硫燃料的燃烧会导致硫氧化物的排放,这对环境有害。加氢脱硫(HDS)工艺是传统的燃料脱硫方法。然而,这种方法成本高,能耗大,因此,替代脱硫方法不断被研究。其中一种燃料脱硫方法是利用深共晶溶剂(DESs)进行溶剂萃取。在本研究中,以氯化胆碱和甘油为基础,配制了一种DES。在DES中加入四种不同的金属氯化物,即氯化铁(III)、氯化铜(II)、氯化锌(II)和氯化锡(II),以提高DES的脱硫效率。用FTIR、流变性、表面张力和Kamlet-Taft参数对溶剂进行了表征。分析了这些溶剂对模型喷气燃料脱硫的效果。为了更好地理解脱硫过程,还对溶剂和模型燃料进行了分子动力学模拟。实验和模拟结果均表明,含氯化铜的DES脱硫效率最高。在溶剂与料料比为1:5、萃取温度为25℃、萃取时间为3 h的条件下,脱硫效率最高,达到99.15%(以总硫为基础)。本文章由计算机程序翻译,如有差异,请以英文原文为准。
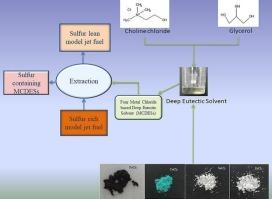
Extractive desulfurization of model jet fuel using metal chloride modified deep eutectic solvents
Crude oil contains various organic sulfur compounds comprising of heterocyclic and paraffinic hydrocarbons. During upgradation of crude oil, these sulfur compounds inevitably end up in the final products, which include different fuel fractions (gasoline, kerosene, diesel, etc.). Jet fuels are one such crude oil fraction. Combustion of high sulfur containing fuels lead to sulfur oxide emissions which is detrimental for the environment. Hydrodesulfurization (HDS) process is the conventional fuel desulfurization approach. However, this method is cost and energy intensive therefore, alternative desulfurization approaches are continually being investigated. One such fuel desulfurization route is solvent extraction utilizing deep eutectic solvents (DESs). In this investigation, a choline chloride and glycerol based DES has been formulated. Four different metal chlorides viz. iron(III) chloride, copper(II) chloride, zinc(II) chloride and tin(II) chloride were added to the DES with an objective to enhance the desulfurization efficiency. These solvents were characterized by FTIR, rheology, surface tension and Kamlet-Taft parameters. The effectiveness of these solvents towards desulfurization of model jet fuel has been analysed. A molecular dynamic simulation of the solvents as well as the model fuel has also been carried out for better understanding of the desulfurization process. Both the experimental and simulated findings indicated that the desulfurization efficiency of the DES containing copper(II) chloride was the maximum. The highest desulfurization efficiency of 99.15 % (on total sulfur basis) with a solvent:feed ratio of 1:5 at an operating temperature of 25 °C and an extraction time of 3 h was achieved using the aforementioned DES.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Molecular Liquids
化学-物理:原子、分子和化学物理
CiteScore
10.30
自引率
16.70%
发文量
2597
审稿时长
78 days
期刊介绍:
The journal includes papers in the following areas:
– Simple organic liquids and mixtures
– Ionic liquids
– Surfactant solutions (including micelles and vesicles) and liquid interfaces
– Colloidal solutions and nanoparticles
– Thermotropic and lyotropic liquid crystals
– Ferrofluids
– Water, aqueous solutions and other hydrogen-bonded liquids
– Lubricants, polymer solutions and melts
– Molten metals and salts
– Phase transitions and critical phenomena in liquids and confined fluids
– Self assembly in complex liquids.– Biomolecules in solution
The emphasis is on the molecular (or microscopic) understanding of particular liquids or liquid systems, especially concerning structure, dynamics and intermolecular forces. The experimental techniques used may include:
– Conventional spectroscopy (mid-IR and far-IR, Raman, NMR, etc.)
– Non-linear optics and time resolved spectroscopy (psec, fsec, asec, ISRS, etc.)
– Light scattering (Rayleigh, Brillouin, PCS, etc.)
– Dielectric relaxation
– X-ray and neutron scattering and diffraction.
Experimental studies, computer simulations (MD or MC) and analytical theory will be considered for publication; papers just reporting experimental results that do not contribute to the understanding of the fundamentals of molecular and ionic liquids will not be accepted. Only papers of a non-routine nature and advancing the field will be considered for publication.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


