壳聚糖-聚乙烯亚胺包封铝-钯层状氢氧化物高效吸附百草枯除草剂的设计与优化
IF 5.2
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
利用环氧氯丙烷作为交联剂,将铝钯层状双氢氧化物(AlPd-LDH)包埋在壳聚糖/聚乙烯亚胺(CS-PEI)框架内,制备了AlPd-LDH/CS-PEI水凝胶珠。这些复合水凝胶珠用于活性捕获阳离子除草剂百草枯(PQ2+)。通过各种技术,包括PXRD, XPS, FESEM, EDX, FT-IR和氮吸附-脱附等温线,对吸附剂的结构和表面特征进行了全面的评估。该研究揭示了一种介孔结构,其表面积为57.54 m2/g。为了研究吸附过程,进行了批量试验,考察了pH、温度、用量和初始百草枯浓度的变化。动力学研究表明,吸附方法符合准二级动力学,平衡数据符合Langmuir等温线模型,为单层吸附。计算的吸附能(34.58 kJ/mol)和热力学极限(ΔG°,ΔH°和ΔS°)表明该过程是自发的吸热过程。对热力学属性的考察揭示了吸附剂和吸附质的显著联系。这些相互作用主要源于物理力,如静电吸引、孔隙填充、π -π堆叠和氢键,而不是共价或配位化学键。这些结果揭示了PQ2+在水凝胶珠上有利和可逆的吸附现象。此外,包括Box-Behnken设计(BBD)和响应面法(RSM)在内的优化技术显著提高了吸附效果,凸显了该复合材料在水净化要求方面的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
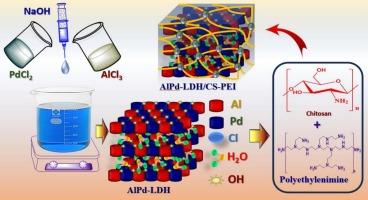
Design and optimization of chitosan–polyethylenimine encapsulated aluminum–palladium layered double hydroxide for efficient paraquat herbicide adsorption via Box–Behnken approach
The AlPd-LDH/CS-PEI hydrogel beads was created by embedding aluminum–palladium layered double hydroxide (AlPd-LDH) within a chitosan/polyethylenimine (CS-PEI) framework, utilizing epichlorohydrin as a cross-linking agent. These composite hydrogel beads were intended for the active capture of the cationic herbicide paraquat (PQ2+). A complete assessment of the structural and surface belongings of the adsorbent was conducted through various techniques, including PXRD, XPS, FESEM, EDX, FT-IR, and nitrogen adsorption–desorption isotherm. The study exposed a mesoporous architecture considered by a surface area measuring 57.54 m2/g. To investigate the adsorption process, batch tests were achieved, examining the belongings of pH, temperature, dosage, and initial paraquat concentration. Kinetic studies indicated that the adsorption method adhered to pseudo-second-order kinetics, while the equilibrium data fit the Langmuir isotherm model, which signifies monolayer adsorption. The calculated adsorption energy (34.58 kJ/mol) and thermodynamic limits (ΔG°, ΔH°, and ΔS°) indicate that the procedure is spontaneous and endothermic. An examination of the thermodynamic belongings reveals notable communications among the adsorbent and the adsorbate. These interactions primarily stem from physical forces such as electrostatic attractions, pore filling, π–π stacking, and hydrogen bonding, as opposed to covalent or coordinative chemical bonds. These results shed light on the favorable and reversible adsorption appearances of PQ2+ onto the hydrogel beads. Furthermore, optimization techniques, including Box–Behnken design (BBD) and response surface methodology (RSM), significantly enhanced adsorption efficacy, highlighting the potential of this composite for water purification requests.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Molecular Liquids
化学-物理:原子、分子和化学物理
CiteScore
10.30
自引率
16.70%
发文量
2597
审稿时长
78 days
期刊介绍:
The journal includes papers in the following areas:
– Simple organic liquids and mixtures
– Ionic liquids
– Surfactant solutions (including micelles and vesicles) and liquid interfaces
– Colloidal solutions and nanoparticles
– Thermotropic and lyotropic liquid crystals
– Ferrofluids
– Water, aqueous solutions and other hydrogen-bonded liquids
– Lubricants, polymer solutions and melts
– Molten metals and salts
– Phase transitions and critical phenomena in liquids and confined fluids
– Self assembly in complex liquids.– Biomolecules in solution
The emphasis is on the molecular (or microscopic) understanding of particular liquids or liquid systems, especially concerning structure, dynamics and intermolecular forces. The experimental techniques used may include:
– Conventional spectroscopy (mid-IR and far-IR, Raman, NMR, etc.)
– Non-linear optics and time resolved spectroscopy (psec, fsec, asec, ISRS, etc.)
– Light scattering (Rayleigh, Brillouin, PCS, etc.)
– Dielectric relaxation
– X-ray and neutron scattering and diffraction.
Experimental studies, computer simulations (MD or MC) and analytical theory will be considered for publication; papers just reporting experimental results that do not contribute to the understanding of the fundamentals of molecular and ionic liquids will not be accepted. Only papers of a non-routine nature and advancing the field will be considered for publication.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


