新型4-喹诺酮衍生物通过内在凋亡通路激活对人转移性三阴性乳腺癌细胞施加细胞毒性
IF 4.7
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
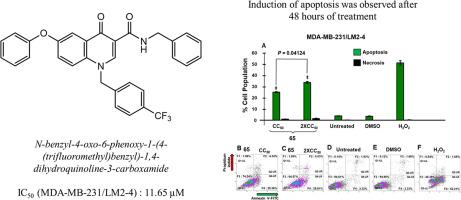
喹诺酮类药物是一类具有抗癌潜力的化合物。然而,目前在临床试验中的喹诺酮类药物目前受到其疗效问题的阻碍。因此,我们设计并合成了新的4-喹诺酮-3-羧酰胺衍生物14-67。所有合成的化合物首先用MTT法筛选其细胞毒性活性,然后用差异核染色(DNS)法评估其对白血病和乳腺癌细胞的抗癌活性。6个化合物对乳腺癌细胞的CC50抑制作用较低,其中化合物24、25、52和65对白血病细胞(Jurkat)也有较好的抑制作用(CC50 = 6.99 ~ 11.17 μM)。体外实验结果表明,活性最高的化合物65在乳腺癌细胞系中具有较高的生长抑制活性(CC50 = 7.10-83.2 μM),而对非癌细胞(mcf - 10a)的细胞毒性较小。在MDA-MB231/LM2-4细胞系中,活性最高的类似物化合物65通过膜去极化触发细胞凋亡,导致细胞周期阻滞在S期。综上所述,化合物65是一种潜在的强效抗癌药物,值得进一步研究和开发。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Novel 4-quinolone derivative inflicted cytotoxicity via intrinsic apoptotic pathway activation on human metastatic triple-negative breast cancer cells
Quinolones are a class of compounds that have shown promising potential as anticancer agents. However, the current quinolones in clinical trials are currently hampered by their efficacy issues. Therefore, we designed and synthesized novel 4-quinolone-3-carboxamide derivatives 14–67. All the synthesized compounds were initially screened for their cytotoxicity activity using the MTT assay method and evaluated for their anticancer activities against leukemia and breast cancer cells using the Differential Nuclear Staining (DNS) assay. Six screened compounds exhibited low CC50 inhibitory effects against breast cancer cell lines, where compounds 24, 25, 52, and 65 also showed good activity (CC50 = 6.99–11.17 μM) against the leukemia cell line (Jurkat). In vitro assessment showed that the most active compound, 65, exhibited significantly higher growth inhibition activity (CC50 = 7.10–83.2 μM) in breast cancer cell lines, with less cytotoxicity observed in non-cancerous cells (MCF-10 A). The most active analog, compound 65, triggered apoptosis via membrane depolarization and caused cell cycle arrest at the S phase in the MDA-MB231/LM2-4 cell line. In conclusion, the outcomes indicate that compound 65 exhibits the potential to be a potent and effective anticancer agent, making it an excellent candidate for further research and development.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioorganic Chemistry
生物-生化与分子生物学
CiteScore
9.70
自引率
3.90%
发文量
679
审稿时长
31 days
期刊介绍:
Bioorganic Chemistry publishes research that addresses biological questions at the molecular level, using organic chemistry and principles of physical organic chemistry. The scope of the journal covers a range of topics at the organic chemistry-biology interface, including: enzyme catalysis, biotransformation and enzyme inhibition; nucleic acids chemistry; medicinal chemistry; natural product chemistry, natural product synthesis and natural product biosynthesis; antimicrobial agents; lipid and peptide chemistry; biophysical chemistry; biological probes; bio-orthogonal chemistry and biomimetic chemistry.
For manuscripts dealing with synthetic bioactive compounds, the Journal requires that the molecular target of the compounds described must be known, and must be demonstrated experimentally in the manuscript. For studies involving natural products, if the molecular target is unknown, some data beyond simple cell-based toxicity studies to provide insight into the mechanism of action is required. Studies supported by molecular docking are welcome, but must be supported by experimental data. The Journal does not consider manuscripts that are purely theoretical or computational in nature.
The Journal publishes regular articles, short communications and reviews. Reviews are normally invited by Editors or Editorial Board members. Authors of unsolicited reviews should first contact an Editor or Editorial Board member to determine whether the proposed article is within the scope of the Journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


