结构引导的olaparib PET探针可以增强PARP-1的精度成像
IF 4.7
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
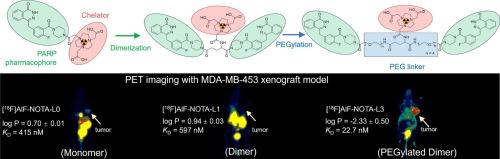
聚(adp -核糖)聚合酶1 (PARP-1)成像在临床肿瘤学中显示出巨大的前景,提供了一种非侵入性的方法来量化肿瘤PARP-1的表达,选择PARP抑制剂治疗的患者,并实时监测治疗反应。然而,目前的parp -1靶向放射性示踪剂是高度亲脂性的,导致肿瘤摄取低,高脱靶积累,成像对比度差,这限制了它们的诊断效用和准确性。为了克服这些限制,我们开发了一系列基于奥拉帕尼药效团的[18F] alf标记的二聚体正电子发射断层扫描(PET)探针,利用多价策略与聚乙二醇化。在候选探针中,[18F]AlF-NOTA-L3 (log P =−2.33±0.50,KD = 75.2 nM)最有希望,在注射后10、30和60分钟分别表现出5.77±0.48、4.21±0.33和3.12±0.19% ID/g的高肿瘤摄取值,相应的肿瘤与肌肉比率为2.75±0.26、10.75±2.16和10.84±3.24,明显优于单体探针[18F]AlF-NOTA-L0。综上所述,[18F]AlF-NOTA-L3具有降低的亲脂性,增强的结合亲和力和优越的肿瘤-背景对比度,显示出提高PARP-1 PET成像用于临床翻译的准确性的巨大潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Structure-guided olaparib PET probe enables enhanced PARP-1 precision imaging
Poly (ADP-ribose) polymerase 1 (PARP-1) imaging shows great promise in clinical oncology, offering a non-invasive way to quantify tumor PARP-1 expression, select patients for PARP inhibitor therapy, and monitor treatment response in real-time. However, current PARP-1-targeted radiotracers are highly lipophilic, leading to low tumor uptake, high off-target accumulation, and poor imaging contrast, which restrict their diagnostic utility and accuracy. To overcome these limitations, we developed a series of [18F]AlF-labeled dimeric positron emission tomography (PET) probes based on the olaparib pharmacophore, leveraging a multivalency strategy with PEGylation. Among the candidates, [18F]AlF-NOTA-L3 (log P = −2.33 ± 0.50, KD = 75.2 nM) emerged as the most promising, exhibiting high tumor uptake values of 5.77 ± 0.48, 4.21 ± 0.33, and 3.12 ± 0.19 %ID/g at 10, 30, and 60 min post-injection, respectively, with corresponding tumor-to-muscle ratios of 2.75 ± 0.26, 10.75 ± 2.16, and 10.84 ± 3.24, which were markedly superior to the monomeric probe [18F]AlF-NOTA-L0. Taken together, [18F]AlF-NOTA-L3 exhibits reduced lipophilicity, enhanced binding affinity, and superior tumor-to-background contrast, demonstrating significant potential to advance the accuracy for PARP-1 PET imaging for clinical translation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioorganic Chemistry
生物-生化与分子生物学
CiteScore
9.70
自引率
3.90%
发文量
679
审稿时长
31 days
期刊介绍:
Bioorganic Chemistry publishes research that addresses biological questions at the molecular level, using organic chemistry and principles of physical organic chemistry. The scope of the journal covers a range of topics at the organic chemistry-biology interface, including: enzyme catalysis, biotransformation and enzyme inhibition; nucleic acids chemistry; medicinal chemistry; natural product chemistry, natural product synthesis and natural product biosynthesis; antimicrobial agents; lipid and peptide chemistry; biophysical chemistry; biological probes; bio-orthogonal chemistry and biomimetic chemistry.
For manuscripts dealing with synthetic bioactive compounds, the Journal requires that the molecular target of the compounds described must be known, and must be demonstrated experimentally in the manuscript. For studies involving natural products, if the molecular target is unknown, some data beyond simple cell-based toxicity studies to provide insight into the mechanism of action is required. Studies supported by molecular docking are welcome, but must be supported by experimental data. The Journal does not consider manuscripts that are purely theoretical or computational in nature.
The Journal publishes regular articles, short communications and reviews. Reviews are normally invited by Editors or Editorial Board members. Authors of unsolicited reviews should first contact an Editor or Editorial Board member to determine whether the proposed article is within the scope of the Journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


