嘧啶-2,4-二酮衍生物作为新型抗病毒SARS-CoV-2 Mpro抑制剂的发现和评价
IF 4.7
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
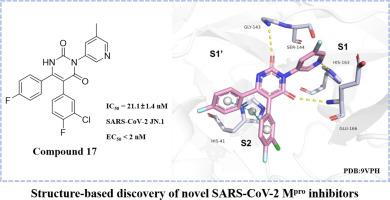
COVID-19大流行凸显了人畜共患冠状病毒传播对全球卫生的持续威胁。在这项研究中,我们以临床验证的SARS-CoV-2主要蛋白酶(Mpro)为目标,采用支架跳跃策略设计和合成了32个嘧啶-2,4-二酮衍生物,旨在探索S1, S1 '和S2子囊中未充分利用的相互作用。结构-活性关系(SAR)分析发现,化合物17是一种有效的Mpro抑制剂(IC50 = 21.1 nM),对SARS-CoV-2 JN.1变体具有显著的抗病毒活性(EC50 < 2 nM)。此外,Mpro-compound 15配合物的x射线晶体学揭示了P1 ' -苯基环与His41之间的t形π-π相互作用。这些结果证明了基于结构优化的嘧啶-2,4-二酮支架在新型冠状病毒Mpro抑制剂开发中的有效性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Discovery and evaluation of pyrimidine-2,4-dione derivatives as novel SARS-CoV-2 Mpro inhibitors with antiviral effect
The COVID-19 pandemic has underscored the persistent threat of zoonotic coronavirus transmission to global health. In this study, we targeted the clinically validated SARS-CoV-2 main protease (Mpro) and implemented a scaffold-hopping strategy to design and synthesize 32 pyrimidine-2,4-dione derivatives, aiming to explore underutilized interactions within the S1, S1’, and S2 subpockets. Structure–activity relationship (SAR) analysis identified compound 17 as a potent Mpro inhibitor (IC50 = 21.1 nM) with outstanding antiviral activity against the SARS-CoV-2 JN.1 variant (EC50 < 2 nM). Furthermore, X-ray crystallography of the Mpro–compound 15 complex unveiled a previously unreported T-shaped π-π interaction between the P1’-phenyl ring and His41. These results demonstrate the effectiveness of structure-based optimization of the pyrimidine-2,4-dione scaffold for the development of novel coronavirus Mpro inhibitors.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioorganic Chemistry
生物-生化与分子生物学
CiteScore
9.70
自引率
3.90%
发文量
679
审稿时长
31 days
期刊介绍:
Bioorganic Chemistry publishes research that addresses biological questions at the molecular level, using organic chemistry and principles of physical organic chemistry. The scope of the journal covers a range of topics at the organic chemistry-biology interface, including: enzyme catalysis, biotransformation and enzyme inhibition; nucleic acids chemistry; medicinal chemistry; natural product chemistry, natural product synthesis and natural product biosynthesis; antimicrobial agents; lipid and peptide chemistry; biophysical chemistry; biological probes; bio-orthogonal chemistry and biomimetic chemistry.
For manuscripts dealing with synthetic bioactive compounds, the Journal requires that the molecular target of the compounds described must be known, and must be demonstrated experimentally in the manuscript. For studies involving natural products, if the molecular target is unknown, some data beyond simple cell-based toxicity studies to provide insight into the mechanism of action is required. Studies supported by molecular docking are welcome, but must be supported by experimental data. The Journal does not consider manuscripts that are purely theoretical or computational in nature.
The Journal publishes regular articles, short communications and reviews. Reviews are normally invited by Editors or Editorial Board members. Authors of unsolicited reviews should first contact an Editor or Editorial Board member to determine whether the proposed article is within the scope of the Journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


