Sesquimustard新型DNA加合物的体外研究及质谱表征。
IF 5.4
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
Sesquimustard (Q)是一种有效的双功能烷基化发泡剂,由于其环境持久性和诱导严重基因毒性作用的能力,是一个重要的毒理学问题。它可以产生多种代谢物,其中一些可以作为生物标志物,它可以广泛地烷基化生物体内的关键大分子,包括DNA。虽然之前的研究主要集中在谷胱甘肽和白蛋白加合物作为Q暴露的回顾性生物标志物,但之前没有研究确定Q的DNA加合物。本研究旨在通过首次使用先进的质谱技术表征Q诱导的DNA加合物来填补这一关键空白。采用液相色谱-高分辨率质谱(hplc - hrms)技术,从q处理过的小牛胸腺DNA中鉴定出三种新的DNA加合物——羟乙基硫代乙基硫代乙基鸟嘌呤(HETETE-Ade)、羟乙基硫代乙基硫代乙基鸟嘌呤(HETETE-Gua)和鸟嘌呤-乙基硫代乙基硫代乙基鸟嘌呤(Gua-ETETE-Gua),并通过高分辨率产物离子谱进行了结构鉴定。利用液相色谱-串联质谱法(LC-MS/MS)在人角质细胞(HaCaT)细胞模型中进一步证实了它们的形成,揭示了加合物信号强度的剂量依赖性增加。与基于蛋白质的生物标志物相比,DNA加合物可能在工作流程简单和直接指示基因毒性损伤方面具有潜在的优势。这项研究首次提供了q诱导DNA加合物的分子洞察力,并为其未来在毒理学评估、法医鉴定和生物标志物开发中的应用奠定了基础。本文章由计算机程序翻译,如有差异,请以英文原文为准。
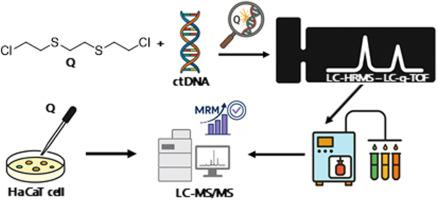
In vitro investigation and mass spectrometric characterization of novel DNA adducts of sesquimustard
Sesquimustard (Q), a potent bifunctional alkylating vesicant, is a significant toxicological concern due to its environmental persistence and capability to induce severe genotoxic effects. It can create several metabolites, some of which can be utilized as biomarkers, and it can extensively alkylate key macromolecules in organisms, including DNA. Although prior research has primarily focused on glutathione and albumin adducts as retrospective biomarkers of Q exposure, no prior studies have identified DNA adducts of Q. This study aimed to fill this critical gap by characterizing, for the first time, Q-induced DNA adducts using advanced mass spectrometric techniques. Three novel DNA adducts -hydroxyethylthioethylthioethyl-adenine (HETETE-Ade), hydroxyethylthioethylthioethyl-guanine (HETETE-Gua), and guanine-ethylthioethylthioethyl-guanine (Gua-ETETE-Gua)- were identified in Q-treated calf thymus DNA via liquid chromatography–high-resolution mass spectrometry (LC-HRMS) and structurally confirmed through high-resolution product ion spectra. Their formation was further demonstrated in a human keratinocyte (HaCaT) cell model using liquid chromatography–tandem mass spectrometry (LC-MS/MS), revealing dose-dependent increases in adduct signal intensities. Compared to protein-based biomarkers, DNA adducts may offer potential advantages in workflow simplicity and direct indication of genotoxic damage. This study provides first molecular insight into Q-induced DNA adducts and lays the groundwork for their future use in toxicological assessment, forensic verification, and biomarker development.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.70
自引率
3.90%
发文量
410
审稿时长
36 days
期刊介绍:
Chemico-Biological Interactions publishes research reports and review articles that examine the molecular, cellular, and/or biochemical basis of toxicologically relevant outcomes. Special emphasis is placed on toxicological mechanisms associated with interactions between chemicals and biological systems. Outcomes may include all traditional endpoints caused by synthetic or naturally occurring chemicals, both in vivo and in vitro. Endpoints of interest include, but are not limited to carcinogenesis, mutagenesis, respiratory toxicology, neurotoxicology, reproductive and developmental toxicology, and immunotoxicology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


