PIM-1通过促进小胶质细胞NLRP3炎性体激活而加剧败血症相关脑病。
IF 8.2
2区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
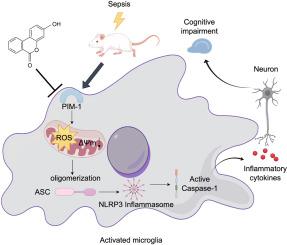
背景:脓毒症相关脑病(SAE)是脓毒症患者中最常见的神经系统并发症之一,导致死亡率增加和长期认知障碍。小胶质线粒体活性氧(mtROS)过量产生和NLR家族pyrin domain-containing 3 (NLRP3)炎性体激活是SAE的关键病理驱动因素。虽然已知PIM-1(一种丝氨酸/苏氨酸激酶)调节mtROS的生成和NLRP3信号传导,但其对SAE的具体贡献仍不明确。方法:本研究采用盲肠结扎穿刺(CLP)手术建立SAE小鼠模型,通过免疫组化、GEO数据库分析等方法筛选差异表达基因PIM-1。采用脂多糖+腺苷(LPS+ATP)三磷酸刺激小鼠小胶质细胞(BV-2)建立体外模型,通过siRNA干扰、RNA-seq等方法分析PIM-1的调控机制。通过分子对接筛选靶向PIM-1抑制的潜在药物尿素B (UB),并通过热稳定性实验、行为实验等评价其对PIM-1的抑制作用和对SAE的治疗潜力。结果:PIM-1在SAE中表达上调,与脑损伤相关。PIM-1敲除抑制小胶质细胞激活,减少炎症细胞因子释放。进一步的研究表明,PIM-1调节mtROS的产生,促进NLRP3炎性体的激活。值得注意的是,UB对PIM-1的药理抑制在体内和体外均减轻了SAE,从而改善了神经元损伤和认知功能障碍。结论:PIM-1可能通过mtROS/NLRP3轴介导小胶质细胞激活和神经炎症,在SAE进展中发挥了关键作用。值得注意的是,尿素B对PIM-1的药理学抑制显著减轻了SAE。本文章由计算机程序翻译,如有差异,请以英文原文为准。
PIM-1 exacerbates sepsis-associated encephalopathy via promoting microglia NLRP3 inflammasome activation
Background
Sepsis-associated encephalopathy (SAE) represents one of the most common neurological complications observed in sepsis patients, contributing to both increased mortality and long-term cognitive impairment. Microglial mitochondrial reactive oxygen species (mtROS) overproduction and NLR family pyrin domain-containing 3 (NLRP3) inflammasome activation were key pathological drivers of SAE. Although PIM-1, a serine/threonine kinase, was known to regulate both mtROS generation and NLRP3 signaling, its specific contribution to SAE remained poorly defined.
Methods
This study established a SAE mouse model using cecal ligation and puncture (CLP) surgery, and screened the differentially expressed gene PIM-1 through immunohistochemistry, GEO database analysis, and etc. An in vitro model was established using Lipopolysaccharide + Adenosine(LPS + ATP) Triphosphate stimulated Mouse Microglia (BV-2), and the regulation mechanism of PIM-1 was analyzed through siRNA interference, RNA-seq, and etc. Potential drug Urolithin B (UB) targeting PIM-1 inhibition was screened through molecular docking, and its inhibitory effect on PIM-1 and therapeutic potential in SAE were evaluated through thermal stability experiments, behavioral experiments, etc.
Results
PIM-1 was upregulated in SAE, which was associated with brain damage. PIM-1 knockdown suppressed microglial activation and reduced inflammatory cytokines release. Further investigation indicated that PIM-1 modulated mtROS generation and facilitated NLRP3 inflammasome activation. Notably, pharmacological inhibition of PIM-1 by UB alleviated SAE both in vivo and in vitro, resulting in improved neuronal damage and cognitive impairment.
Conclusion
PIM-1 played a critical role in SAE progression, potentially through its mediation of microglial activation and neuroinflammation via the mtROS/NLRP3 axis. Notably, pharmacological inhibition of PIM-1 with urolithin B significantly alleviated SAE.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Free Radical Biology and Medicine
医学-内分泌学与代谢
CiteScore
14.00
自引率
4.10%
发文量
850
审稿时长
22 days
期刊介绍:
Free Radical Biology and Medicine is a leading journal in the field of redox biology, which is the study of the role of reactive oxygen species (ROS) and other oxidizing agents in biological systems. The journal serves as a premier forum for publishing innovative and groundbreaking research that explores the redox biology of health and disease, covering a wide range of topics and disciplines. Free Radical Biology and Medicine also commissions Special Issues that highlight recent advances in both basic and clinical research, with a particular emphasis on the mechanisms underlying altered metabolism and redox signaling. These Special Issues aim to provide a focused platform for the latest research in the field, fostering collaboration and knowledge exchange among researchers and clinicians.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


