杜伐单抗联合卡铂和依托泊苷治疗晚期肺大细胞神经内分泌癌的开放标签前瞻性II期研究方案(LOGIK2401: NECTAR研究)。
IF 2.4
Q3 Medicine
引用次数: 0
摘要
简介:肺大细胞神经内分泌癌(Large cell neuroendocrine carcinoma, LCNEC)是一种罕见的组织学类型,在最新的组织学分类中与小细胞肺癌(small cell lung cancer, SCLC)并列在神经内分泌肿瘤范畴。化疗和免疫检查点抑制剂联合治疗是非小细胞肺癌和SCLC的标准治疗。然而,免疫化疗对LCNEC的疗效尚未确定。方法和分析:这项前瞻性、多中心、单臂研究将评估杜伐单抗联合卡铂和依托泊苷治疗晚期或复发LCNEC患者的疗效。30名患者参加了这项研究。患者将接受杜伐单抗静脉注射联合至多4个周期的卡铂和依托泊苷,随后进行杜伐单抗维持治疗。主要终点是客观缓解率,关键次要终点是缓解持续时间、无进展生存期、总生存期和安全性。结论:这是一项评估晚期或复发LCNEC患者免疫化疗疗效的前瞻性研究。研究结果将有助于建立LCNEC的治疗策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
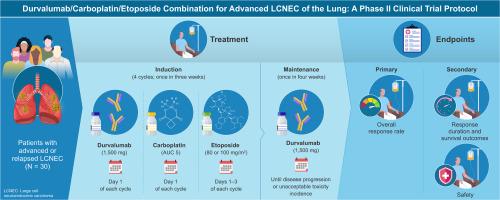
Study protocol of an open-label prospective phase II study of Durvalumab plus Carboplatin and Etoposide in advanced large cell neuroendocrine carcinoma of the lung (LOGIK2401: NECTAR study)
Introduction
Large cell neuroendocrine carcinoma (LCNEC) of the lung is a rare histologic type and is placed in the neuroendocrine tumor category along with small cell lung cancer (SCLC) at the latest histologic classification. Combination therapy with chemotherapy and immune checkpoint inhibitors is the standard of care for both non-small cell lung cancer and SCLC. However, the efficacy of immunochemotherapy for LCNEC has not yet been established.
Methods and analysis
This prospective, multicenter, single-arm study will evaluate the efficacy of durvalumab plus carboplatin and etoposide in patients with advanced or relapsed LCNEC. Thirty patients were enrolled in this study. Patients will receive durvalumab intravenously combined with up to four cycles of carboplatin and etoposide, followed by durvalumab maintenance treatment. The primary endpoint is the objective response rate, and the key secondary endpoints are the duration of response, progression-free survival, overall survival, and safety.
Conclusion
This is the prospective study to evaluate the efficacy of immunochemotherapy in patients with advanced or relapsed LCNEC. The results are expected to contribute to establishing the treatment strategy for LCNEC.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cancer treatment and research communications
Medicine-Oncology
CiteScore
4.30
自引率
0.00%
发文量
148
审稿时长
56 days
期刊介绍:
Cancer Treatment and Research Communications is an international peer-reviewed publication dedicated to providing comprehensive basic, translational, and clinical oncology research. The journal is devoted to articles on detection, diagnosis, prevention, policy, and treatment of cancer and provides a global forum for the nurturing and development of future generations of oncology scientists. Cancer Treatment and Research Communications publishes comprehensive reviews and original studies describing various aspects of basic through clinical research of all tumor types. The journal also accepts clinical studies in oncology, with an emphasis on prospective early phase clinical trials. Specific areas of interest include basic, translational, and clinical research and mechanistic approaches; cancer biology; molecular carcinogenesis; genetics and genomics; stem cell and developmental biology; immunology; molecular and cellular oncology; systems biology; drug sensitivity and resistance; gene and antisense therapy; pathology, markers, and prognostic indicators; chemoprevention strategies; multimodality therapy; cancer policy; and integration of various approaches. Our mission is to be the premier source of relevant information through promoting excellence in research and facilitating the timely translation of that science to health care and clinical practice.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


