优化吡咯-2-羧酸酰胺制备具有较好理化性能和可用药性的强效抗结核药物。
IF 4.7
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
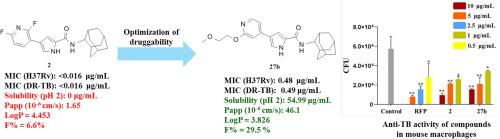
MmpL3是分枝杆菌的一种膜蛋白,对海藻糖单菌酸盐的运输至关重要,海藻糖单菌酸盐对结核分枝杆菌外膜的形成和细菌的存活至关重要。在此,我们优化了我们的先导MmpL3抑制剂承载吡咯-2-羧酰胺支架,以开发具有改进的物理化学性能的抗结核药物。化合物27b是我们的主要MmpL3抑制剂的优化类似物,具有增强的抗结核活性,降低细胞毒性,改善微粒体稳定性和高caco2渗透性。与先导化合物2相比,化合物27b的水溶性和药代动力学特征明显改善。该化合物在降低小鼠巨噬细胞内细胞内结核分枝杆菌负荷方面表现出强有力的功效。本研究结果表明,在吡咯-2-羧酸酰胺支架中加入含氧基团可以提高化合物的LogP值,从而在亲脂性和抗结核活性之间取得平衡。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Optimization of pyrrole-2-carboxamide to develop a potent antituberculosis agent with improved physicochemical property and druggability
MmpL3, a mycobacterial membrane protein, is essential for the transport of trehalose monomycolate, which is crucial for the formation of the M. tuberculosis outer membrane and the survival of the bacterium. Herein, we optimize our lead MmpL3 inhibitor bearing pyrrole-2-carboxamide scaffold to develop antituberculosis agents with improved physicochemical properties. Compound 27b, an optimized analog of our lead MmpL3 inhibitor, exhibited enhanced antituberculosis activity along with reduced cytotoxicity, improved microsomal stability, and high Caco-2 permeability. Significantly, the water solubility and pharmacokinetic profile of compound 27b was markedly improved compared to the lead compound 2. This compound demonstrated potent efficacy in decreasing the intracellular M. tuberculosis load within mouse macrophages. The results of this study indicated that incorporating an oxygen-containing group in pyrrole-2-carboxamide scaffold can improve the compound's LogP value, thereby achieving a balance between lipophilicity and antituberculosis activity.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioorganic Chemistry
生物-生化与分子生物学
CiteScore
9.70
自引率
3.90%
发文量
679
审稿时长
31 days
期刊介绍:
Bioorganic Chemistry publishes research that addresses biological questions at the molecular level, using organic chemistry and principles of physical organic chemistry. The scope of the journal covers a range of topics at the organic chemistry-biology interface, including: enzyme catalysis, biotransformation and enzyme inhibition; nucleic acids chemistry; medicinal chemistry; natural product chemistry, natural product synthesis and natural product biosynthesis; antimicrobial agents; lipid and peptide chemistry; biophysical chemistry; biological probes; bio-orthogonal chemistry and biomimetic chemistry.
For manuscripts dealing with synthetic bioactive compounds, the Journal requires that the molecular target of the compounds described must be known, and must be demonstrated experimentally in the manuscript. For studies involving natural products, if the molecular target is unknown, some data beyond simple cell-based toxicity studies to provide insight into the mechanism of action is required. Studies supported by molecular docking are welcome, but must be supported by experimental data. The Journal does not consider manuscripts that are purely theoretical or computational in nature.
The Journal publishes regular articles, short communications and reviews. Reviews are normally invited by Editors or Editorial Board members. Authors of unsolicited reviews should first contact an Editor or Editorial Board member to determine whether the proposed article is within the scope of the Journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


