在环境条件下通过质子耦合的原位辐射分解电子转移从氮和水有效地生产氨
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
氨(NH3)是维持全球粮食供应和燃料生产的重要组成部分,但其通过哈伯-博世工艺进行的工业生产仍然需要大量能源和碳。在这里,我们报告了一种在环境条件下仅利用大气N2和水作为原料的电子束激活NH3合成策略。该方法实现了83.6 μmol g-1 s-1的工业相关速率,大大提高了0.53 MJ/mol的能量效率,超过了大多数实验室规模的技术,与Haber-Bosch工艺相当。时间分辨operando光谱实验,结合理论计算,揭示了这种独特的激活模式能够通过质子耦合转移原位产生的高能水合电子实现N2→NH3的快速转化。此外,技术经济分析和生命周期评估表明,目前的固氮方法操作简单,产量高,可以促进可持续化工行业的氮循环。本文章由计算机程序翻译,如有差异,请以英文原文为准。
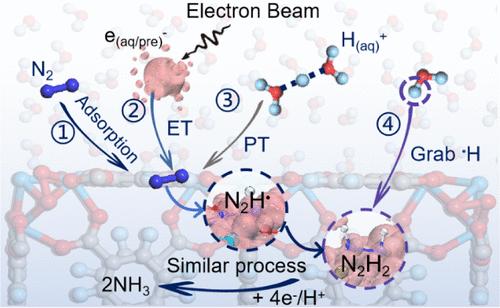
Effective Ammonia Production from Nitrogen and Water under Ambient Conditions through Proton-Coupled In Situ Radiolytic Electron Transfer
Ammonia (NH3) is a vital component for sustaining global food supplies and fuel production, but its industrial production via the Haber–Bosch process remains energetically demanding and carbon-intensive. Here, we report an electron-beam-activated NH3 synthesis strategy that utilizes only atmospheric N2 and water as feedstocks under ambient conditions. This approach achieves an industrially relevant rate of 83.6 μmol g–1 s–1 with a substantially improved energy efficiency of 0.53 MJ/mol, which surpasses most laboratory-scale technologies and is comparable to that of the Haber–Bosch process. Time-resolved operando spectroscopic experiments, combined with theoretical calculations, reveal that this distinct activation mode enables rapid N2→NH3 conversion through a proton-coupled transfer of an in situ generated energetic hydrated electron. Furthermore, techno-economic analysis and life-cycle assessment demonstrate that the operational simplicity and exceptional throughput of the present N2 fixation approach could promote nitrogen circularity in the sustainable chemical industry.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


