精确合成外消旋酸基单手螺旋聚合物作为多重不对称反应的可回收均相手性催化剂
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
从外消旋体材料中开发手性催化剂,同时保留均相和非均相催化剂的优点,是迄今为止一个非常理想但艰巨的挑战。在这项工作中,我们用手性钯(II)催化剂从外消旋单体合成了具有光学活性的单手螺旋聚碳烯。用手性Pd(II)/LR或S催化剂配以R-或S-双齿膦配体,聚合含有叔丁基羧基保护的L-脯氨酸醇酯的重氮乙酸酯对映体(L-和D-1),具有较高的对映选择性(kfast/kslow = 142)。有趣的是,外消旋D/L-1的活性聚合在Pd(II)/LR和S催化剂下分别表现出较高的螺旋选择性,并产生左旋和右旋螺旋。消去脯氨酸酯上保护叔丁基羧基的外消旋基螺旋聚碳胺衍生物在不对称Aldol反应、Michael加成反应和Domino oxa-Michael/Aldol缩合反应中表现出良好的催化活性和对映选择性。所有的反应都能得到高收率的目标产物(>78%),对映体过量(ee)高达99%。令人惊讶的是,对映体选择性完全取决于主链的螺旋度,而不考虑其手性;因此,使用相反手性的螺旋聚合物基催化剂很容易得到对映体产物。聚合物催化剂可以通过溶剂沉淀从均相反应溶液中回收,并且在保持良好的活性和对映体选择性的同时,至少可循环使用5次,证实了这些外消旋酸基螺旋聚合物具有均相和非均相催化剂的优点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
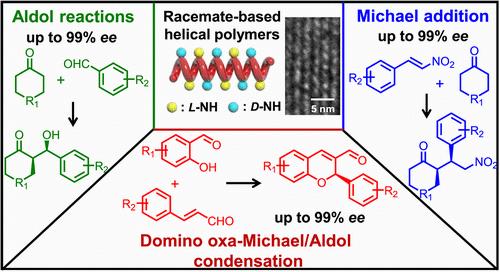
Precise Synthesis of Racemate-Based One-Handed Helical Polymers as Recyclable Homogeneous Chiral Catalysts for Multiple Asymmetric Reactions
Developing chiral catalysts from racemic materials while retaining the merits of both homogeneous and heterogeneous catalysts is a highly desirable yet formidable challenge to date. In this work, we synthesized optically active, one-handed helical polycarbenes from racemic monomers using chiral Pd(II) catalysts. The living polymerization of enantiomeric diazo acetate monomers (L- and D-1) bearing tert-butyloxycarboryl-protected L-prolinol ester using chiral Pd(II)/LR,or,S catalysts coordinated with R- or S-bidentate phosphine ligand showed high enantioselectivity (kfast/kslow = 142). Interestingly, the living polymerization of racemic D/L-1 shows high helix-sense selectivity and affords left- and right-handed helices by using Pd(II)/LR,and,S catalysts, respectively. Removing the tert-butyloxycarboryl-protected groups on prolinol esters, the racemate-based helical polycarbenes bearing amine pendants showed excellent catalytic activity and enantioselectivity in asymmetric Aldol reaction, Michael addition, and Domino oxa-Michael/Aldol condensation. All of the reactions gave the target products in high yields (>78%) with enantiomeric excess (ee) up to 99%. Surprisingly, enantioselectivity is solely determined by the backbone helicity, regardless of the pendant chirality; thus, enantiomeric products are easily obtained using helical polymer-based catalysts in opposite handedness. The polymer catalysts could be recovered from the homogeneous reaction solution via solvent precipitation and were recycled at least 5 times while maintaining excellent activity and enantioselectivity, confirming that these racemate-based helical polymers have the merits of both homogeneous and heterogeneous catalysts.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


