早期肥胖相关胰岛素抵抗的分子特征:脂肪细胞来源的细胞外囊泡蛋白揭示了代谢功能障碍的阶段特异性候选物。
IF 2.8
2区 生物学
Q2 BIOCHEMICAL RESEARCH METHODS
引用次数: 0
摘要
内脏型肥胖与胰岛素抵抗密切相关,胰岛素抵抗是代谢性疾病发生的关键过程。脂肪细胞来源的细胞外囊泡(addev)作为细胞间通讯的介质,携带反映脂肪组织功能状态的信号。本研究旨在对addev进行比较蛋白质组学分析,以提出早发性肥胖IR特异性生物标志物候选物的分子指纹图谱。从高脂饮食(HFD)和对照组的16周龄雄性Wistar大鼠附睾脂肪细胞中分离出AdEVs,并通过质谱联用多软件蛋白质鉴定生物信息学策略进行分析。对于使用无标记方法的相对定量,鉴定了超过1200种蛋白质,其中431种被过度代表并且是HFD所特有的,与能量代谢,细胞应激和胰岛素信号传导明确相关。基于生物学合理性和/或最佳log2FC和p值评分,提出了6种蛋白质作为IR分子指纹图谱的一部分:Atp5f1b、Anxa6、Myo1c、GLUT4、Anxa5和Aoc3。磷酸化蛋白组学分析揭示了CaATPase (S663)、PLIN (S130)和PPM1H (S260,265)等磷酸化蛋白的关键修饰。结果表明,addev反映了与IR相关的早期线粒体改变,可作为潜在的非侵入性生物标志物候选物,用于早期超重或肥胖的代谢功能障碍和随访。在这项研究中,我们证明了脂肪细胞衍生的细胞外囊泡(addev)包含了一个阶段分解的分子特征,反映了肥胖症胰岛素抵抗早期发展的关键病理生理事件。这些囊泡携带与脂肪毒性、线粒体和内质网应激以及囊泡运输受损相关的蛋白质,为局部脂肪细胞功能障碍如何引发全身代谢损伤提供了机制见解。这种基于ev的蛋白质组学指纹图谱促进了我们对胰岛素抵抗发病机制的理解,并确定了早期代谢风险分层的潜在生物标志物候选物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
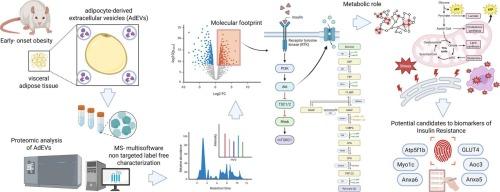
Molecular signature of early obesity-associated insulin resistance: Adipocyte-derived extracellular vesicle proteins reveal stage-specific candidates for metabolic dysfunction
Visceral obesity is closely related to insulin resistance (IR), a key process in developing metabolic diseases. Adipocyte-derived extracellular vesicles (AdEVs) have emerged as mediators of intercellular communication, carrying signals reflecting adipose tissue's functional state. This study aimed to perform a comparative proteomic analysis of AdEVs to propose a molecular fingerprint of specific biomarker candidates for IR in early-onset obesity. AdEVs were isolated from epididymal adipocytes of 16-week-old male Wistar rats on a high-fat diet (HFD) and controls and analyzed by mass spectrometry coupled to a multi-software protein identification bioinformatics strategy. For relative quantification using the label-free method, more than 1200 proteins were identified, with 431 being overrepresented and unique to HFD, associated explicitly with energy metabolism, cellular stress, and insulin signaling. Based on biological plausibility, and/or the best log2FC and p-value scores, six proteins were proposed as part of the IR molecular fingerprint: Atp5f1b, Anxa6, Myo1c, GLUT4, Anxa5, and Aoc3. Phosphoproteomic analysis revealed key modifications in phosphorylated proteins such as CaATPase (S663), PLIN (S130), and PPM1H (S260,265). The results suggest that AdEVs reflect early mitochondrial alterations related to IR, which could be employed as potential non-invasive biomarker candidates for metabolic dysfunction and follow-up in early overweight or obesity.
Significance
In this study, we demonstrate that adipocyte-derived extracellular vesicles (AdEVs) encapsulate a stage-resolved molecular signature that reflects key pathophysiological events underlying the early development of insulin resistance in obesity. These vesicles carry proteins linked to lipotoxicity, mitochondrial and endoplasmic reticulum stress, and impaired vesicular trafficking, offering mechanistic insight into how local adipocyte dysfunction may trigger systemic metabolic impairment. This EV-based proteomic fingerprint advances our understanding of insulin resistance pathogenesis and identifies potential biomarker candidates for early metabolic risk stratification.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of proteomics
生物-生化研究方法
CiteScore
7.10
自引率
3.00%
发文量
227
审稿时长
73 days
期刊介绍:
Journal of Proteomics is aimed at protein scientists and analytical chemists in the field of proteomics, biomarker discovery, protein analytics, plant proteomics, microbial and animal proteomics, human studies, tissue imaging by mass spectrometry, non-conventional and non-model organism proteomics, and protein bioinformatics. The journal welcomes papers in new and upcoming areas such as metabolomics, genomics, systems biology, toxicogenomics, pharmacoproteomics.
Journal of Proteomics unifies both fundamental scientists and clinicians, and includes translational research. Suggestions for reviews, webinars and thematic issues are welcome.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


