(±)-拉帕内酯A和B的仿生全合成。
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
利用仿生的vinylogi - michael -oxa- michael多米诺骨牌反应,分两步合成了第一个全脂内酯A和B。在1,2- dce中与Cs2CO3在高温下进行多米诺反应,需要仔细的动力学控制。全合成进一步证明了上盖内酯是天然产物虹膜苷二聚体的生物合成假说。本文章由计算机程序翻译,如有差异,请以英文原文为准。
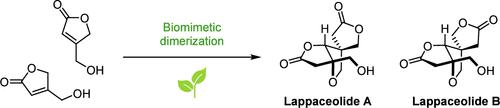
Biomimetic Total Synthesis of (±)-Lappaceolides A and B.
The first total synthesis of lappaceolides A and B is achieved in two steps by using a biomimetic vinylogous-Michael-oxa-Michael domino reaction. The domino reaction proceeds with Cs2CO3 in 1,2-DCE at elevated temperatures and requires careful kinetic control. The total synthesis provides further proof of the biosynthetic hypothesis of lappaceolides being dimers of the natural product siphonodin.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


