嵌入配体的光开关PROTAC用于微管蛋白的时空降解。
IF 3
3区 医学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
本研究通过整合偶氮苯修饰的combretastatin A4 (Azo-CA4)作为光可控微管蛋白配体,开发了一种新的光开关蛋白水解靶向嵌合体(PROTAC)。与传统的光调节PROTACs不同,我们的策略是将光开关直接嵌入靶蛋白配体(偶氮- ca4)中。在365 nm紫外光下,偶氮- ca4异构成顺式构型,实现高亲和的微管蛋白结合和随后的泛素-蛋白酶体依赖性降解。先导化合物AC2对三阴性乳腺癌(MDA-MB-231细胞)表现出明显的光依赖性抗肿瘤活性,其效力增强了15倍(IC₅0 = 4.05±0.13 μM,紫外线下为63.64 μM,黑暗中为63.64 μM)。此外,AC2在光照和黑暗条件下(IC₅₀> 100 μM)对正常乳腺上皮细胞(MCF-10A)都表现出最小的毒性,突出了其良好的选择性。机制分析建立了可逆的β-微管蛋白降解,泛素-蛋白酶体系统(UPS)依赖性(被MG132抑制)和强大的三元配合物形成(结合能:-5.96 kcal/mol)。ADMET分析显示膜渗透性中等(Log Po/w = 3.19),但由于其高分子量(645 Da)和溶解度差,这种渗透性限制了口服生物利用度。这种配体嵌入方法提高了时空精度,同时减轻了脱靶毒性,为靶向癌症治疗建立了一种新的治疗范式。本文章由计算机程序翻译,如有差异,请以英文原文为准。
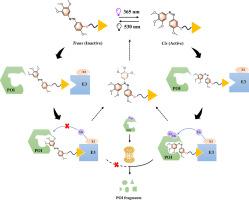
Ligand-embedded photoswitching PROTAC for spatiotemporal tubulin degradation
This study developed a novel light-switchable proteolysis-targeting chimera (PROTAC) by integrating azobenzene-modified combretastatin A4 (Azo-CA4) as a photocontrollable tubulin ligand. In contrast to conventional light-regulated PROTACs that modulate linker conformation, our strategy embeds the photoswitch directly within the target protein ligand (Azo-CA4). Under 365 nm UV light, Azo-CA4 isomerizes to its cis-configuration, enabling high-affinity tubulin binding and subsequent ubiquitin-proteasome-dependent degradation. The lead compound AC2 exhibited pronounced light-dependent antitumor activity against triple-negative breast cancer (MDA-MB-231 cells), with a 15-fold enhancement in potency (IC₅₀ = 4.05 ± 0.13 μM under UV vs. 63.64 μM in the dark). Furthermore, AC2 exhibited minimal toxicity in normal breast epithelial cells (MCF-10A) under both light and dark conditions (IC₅₀ > 100 μM), highlighting its favorable selectivity. Mechanistic analyses established reversible β-tubulin degradation, ubiquitin-proteasome system (UPS) dependency (inhibited by MG132), and robust ternary complex formation (binding energy: −5.96 kcal/mol). ADMET profiling indicated moderate membrane permeability (Log Po/w = 3.19) but this permeability limited oral bioavailability, attributable to its high-molecular-weight (645 Da) and poor solubility. This ligand-embedded approach enhances spatiotemporal precision while mitigating off-target toxicity, establishing a novel therapeutic paradigm for targeted cancer therapy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioorganic & Medicinal Chemistry
医学-生化与分子生物学
CiteScore
6.80
自引率
2.90%
发文量
413
审稿时长
17 days
期刊介绍:
Bioorganic & Medicinal Chemistry provides an international forum for the publication of full original research papers and critical reviews on molecular interactions in key biological targets such as receptors, channels, enzymes, nucleotides, lipids and saccharides.
The aim of the journal is to promote a better understanding at the molecular level of life processes, and living organisms, as well as the interaction of these with chemical agents. A special feature will be that colour illustrations will be reproduced at no charge to the author, provided that the Editor agrees that colour is essential to the information content of the illustration in question.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


