喹啉-铬烯复合物作为双拓扑异构酶抑制剂的合成、硅和体外评价。
IF 3
3区 医学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
喹啉和铬支架具有公认的抗癌活性,但其协同作用尚未得到广泛研究。我们目前的工作研究了选择性设计的一系列喹啉-铬杂合体,采用综合的方法结合计算和实验评估。对拓扑异构酶I (3QX3)和II (4FM9)进行的分子对接实验表明,该酶与铅分子6c、6l和6j具有较高的结合亲和力,与标准试剂喜树碱和蜜柑碱相比,其疗效更高。此外,分子动力学模拟证实了配体与蛋白质配合物的稳定性,RMSD值较低,MM-GBSA结合自由能为正。ADMET分析预测了高口服生物利用度,代谢稳定性和可耐受的毒性水平,并鼓励药物样行为。这些杂种中的7个在几种癌细胞系(HepG2, Hep3B, HCT-116和MCF-7)中进行了细胞毒性实验评估,并显示出与正常细胞(HEK-293细胞)相关的选择性行为。值得注意的是,化合物6c和6l显示出亚微摩尔IC₅0值和强的双拓扑异构酶I/II抑制作用,并验证了它们的作用方式。构效关系(SAR)分析表明,具有给电子作用的取代基增加了π-π堆积和氢键,并与增强的效价和选择性密切相关。总的来说,这些结果将喹啉-铬烯杂合体定位为抗癌治疗的重要线索,并强调了支架杂交在实现高效、选择性和机制建立的拓扑异构酶抑制方面的优势。本文章由计算机程序翻译,如有差异,请以英文原文为准。
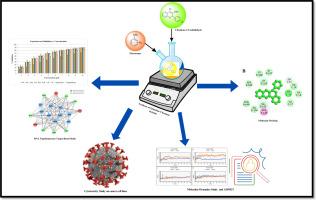
Synthesis, in-silico and in-vitro evaluation of quinoline-chromene hybrids as dual topoisomerase inhibitors
Quinoline and chromene scaffold are recognized to possess anticancer activities but their synergistic potential has never been studied extensively. Our present work investigated a selectively designed series of quinoline-chromene hybrids using an integrative approach combining computational and experimental evaluations. Molecular docking experiments performed on topoisomerase I (3QX3) and II (4FM9) showed high binding affinities with lead molecules 6c, 6l, and 6j having greater efficacy in comparison to standard agents camptothecin and amsacrine. Moreover, molecular dynamics simulations confirmed the stability of the complexes of the ligand and the protein with low RMSD values and positive MM-GBSA binding free energies. ADMET profiling predicted high oral bioavailability, metabolic stability, and tolerable levels of toxicity with encouraging drug-like behavior. Seven of these hybrids were experimentally assessed for cytotoxicity in several cancer cell lines (HepG2, Hep3B, HCT-116, and MCF-7) and showed selective behavior in relation to normal cells (HEK-293 cells). Notably, the compounds 6c and 6l showed sub-micromolar IC₅₀ values and strong dual topoisomerase I/II inhibition and verified their mode of action. Structure-activity relationship (SAR) analysis showed that substituents with an electron-donating effect increased π–π stacking and hydrogen bonding and associated well with enhanced potency and selectivity. Collectively, these results position quinoline–chromene hybrids as important leads in anticancer treatment and emphasize the advantages of scaffold hybridization in attaining efficient, selective, and mechanistically established inhibition of the topoisomerase.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioorganic & Medicinal Chemistry
医学-生化与分子生物学
CiteScore
6.80
自引率
2.90%
发文量
413
审稿时长
17 days
期刊介绍:
Bioorganic & Medicinal Chemistry provides an international forum for the publication of full original research papers and critical reviews on molecular interactions in key biological targets such as receptors, channels, enzymes, nucleotides, lipids and saccharides.
The aim of the journal is to promote a better understanding at the molecular level of life processes, and living organisms, as well as the interaction of these with chemical agents. A special feature will be that colour illustrations will be reproduced at no charge to the author, provided that the Editor agrees that colour is essential to the information content of the illustration in question.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


