新一代广谱抗真菌剂N′-(乙基水杨酸基)聚羧酸肼的设计、合成及SAR研究。
IF 3
3区 医学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
本研究探讨了溴是否可以在不失去对真菌的效价和选择性的情况下被其生物等构取代,并发现生物等构替代策略可以成功地应用于新型的第二代含乙基的N'-(水杨基)芳香羰基肼的设计、合成和SAR研究。本研究特别关注对三种关键优先真菌病原体,新形假丝酵母菌,白色假丝酵母菌和烟状假丝酵母菌的广谱抗真菌活性,以选择极具前景的先导化合物。可靠的QSAR模型优化和进一步的药物开发已成功创建使用AutoQSAR程序。此外,进行了计算机ADME/Tox预测,其预测ClogP值显著提高(3.26-5.66),31个化合物中除2个(低置信)化合物外无致突变性(AMES试验),无hERG毒性,无致死毒性(LD50 > 2 Mol/kg), 31个化合物中除2个化合物外无肝毒性,Caco2渗透性良好。本研究确定了3个化合物,8.1,8.6和8.23,作为进一步药物开发的最有希望的线索,它们对3种真菌病原体具有良好的广谱抗真菌活性,具有高(SI 100)至极高(SI高达17,066)的选择性指数和相关的ClogP值(3.26-4.40)。化合物8.1和8.23与3种临床抗真菌药物均有协同作用,尤其是8.1与3种临床抗真菌药物均有协同作用。化合物8.23将作为开发一系列新的无溴N′-(水杨基)芳烃(或杂芳烃)碳酰肼的骨架。本文章由计算机程序翻译,如有差异,请以英文原文为准。
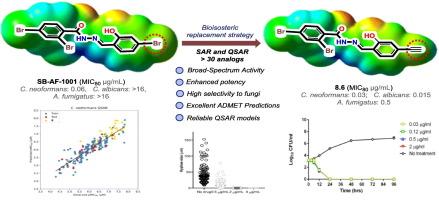
Design, synthesis and SAR studies of N′-(Ethynylsalicylidene)arenecarbohydrazides as next-generation broad-spectrum antifungal agents
The present study investigated whether bromine could be replaced with its bioisostere, ethynyl group, without losing potency and selectivity toward fungi, and has found that the bioisosteric replacement strategy is successfully applied to the design, synthesis and SAR study of novel 2nd-generation N′-(salicylidene)arenecarbohydrazides, bearing ethynyl groups. This study particularly focused on the broad-spectrum antifungal activities against three critical priority fungal pathogens, C. neoformans, C. albicans, and A. fumigatus to select highly promising lead compounds. Reliable QSAR models for optimization and further drug development have been successfully created using the AutoQSAR program. Also, in silico ADME/Tox predictions were performed, which predicted considerably improved ClogP values (3.26–5.66), no mutagenicity (AMES test) except for 2 compounds (with low confidence) out of 31, no hERG toxicity, no lethal toxicity (LD50 > 2 Mol/kg), no hepatotoxicity except for 2 compounds out of 31, and good Caco2 permeability. This study has identified 3 compounds, 8.1, 8.6 and 8.23 as the most promising leads for further drug development, which exhibited excellent broad-spectrum antifungal activities against the three fungal pathogens with high (SI >100) to extremely high (SI up to 17,066) selectivity indices and relevant ClogP values (3.26–4.40). Compounds 8.1 and 8.23 showed synergy in combination with 3 clinical antifungal drugs, especially 8.1 exhibited synergy with 3 clinical drugs against all 3 distinctly different fungal pathogens. Compound 8.23 will serve as the scaffold for developing a new series of bromine-free N′-(salicylidene)arene(or heteroarene)carbohydrazides.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioorganic & Medicinal Chemistry
医学-生化与分子生物学
CiteScore
6.80
自引率
2.90%
发文量
413
审稿时长
17 days
期刊介绍:
Bioorganic & Medicinal Chemistry provides an international forum for the publication of full original research papers and critical reviews on molecular interactions in key biological targets such as receptors, channels, enzymes, nucleotides, lipids and saccharides.
The aim of the journal is to promote a better understanding at the molecular level of life processes, and living organisms, as well as the interaction of these with chemical agents. A special feature will be that colour illustrations will be reproduced at no charge to the author, provided that the Editor agrees that colour is essential to the information content of the illustration in question.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


