酮abno:有机合成与蛋白质修饰的交叉点
IF 7.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
在2012年,我们报道了n -氧自由基ketoABNO作为一种有效的催化剂,可以将胺轻度有氧氧化为亚胺(T. Sonobe, K. Oisaki和M. Kanai, Chem.)。科学。[j], 2012, 3, 3249, https://doi.org/10.1039/C2SC20699D)。它的催化多功能性源于其独特的空间紧致性、高氧化电位以及在三种氧化态(羟胺、n -氧和氧铵)之间可逆相互转化的能力。除了胺氧化,酮abno也被应用于醇和醛的氧化。最近,它的用途已经从小分子转化扩展到蛋白质修饰,例如蛋白质的丝氨酸选择性氧化切割(与水溶性铜络合物催化剂结合)和色氨酸选择性生物偶联。在这篇评论中,我们重点介绍了酮abno作为氧化催化剂的发展及其在生物相容性蛋白质化学中的新应用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
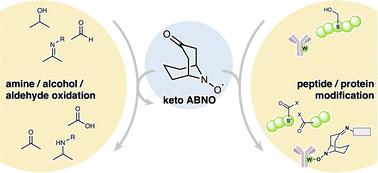
A reflection on ketoABNO: the crossing point between organic synthesis and protein modification
In 2012, we reported that the N-oxyl radical ketoABNO functions as an effective catalyst for the mild aerobic oxidation of amines to imines (T. Sonobe, K. Oisaki and M. Kanai, Chem. Sci., 2012, 3, 3249, https://doi.org/10.1039/C2SC20699D). Its catalytic versatility arises from a unique combination of steric compactness, high oxidation potential, and the ability to reversibly interconvert among three oxidation states—hydroxyamine, N-oxyl, and oxoammonium. Beyond amine oxidation, ketoABNO has also been applied to the oxidation of alcohols and aldehydes. More recently, its utility has extended beyond small-molecule transformations to include applications in protein modifications, such as serine-selective oxidative cleavage of proteins (in conjunction with a water-soluble copper-complex catalyst) and tryptophan-selective bioconjugation. In this Commentary, we highlight the development of ketoABNO as an oxidation catalyst and its emerging applications in biocompatible protein chemistry.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Science
CHEMISTRY, MULTIDISCIPLINARY-
CiteScore
14.40
自引率
4.80%
发文量
1352
审稿时长
2.1 months
期刊介绍:
Chemical Science is a journal that encompasses various disciplines within the chemical sciences. Its scope includes publishing ground-breaking research with significant implications for its respective field, as well as appealing to a wider audience in related areas. To be considered for publication, articles must showcase innovative and original advances in their field of study and be presented in a manner that is understandable to scientists from diverse backgrounds. However, the journal generally does not publish highly specialized research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


