基于物理建模的基于mea的CO2电解槽质量和热管理策略
IF 2.9
3区 化学
Q3 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
基于膜电极组件(MEA)的CO2电解槽由于其紧凑的设计和高电流密度,在电化学CO2还原(CO2R)方面具有很大的前景。然而,性能和耐久性通常受到质量传输限制、热梯度和盐沉淀的限制。我们提出了一个全面的、非等温的、基于物理的模型,该模型捕捉了气体、液体和离子的多相传输,以及热量的产生、电化学反应和基于mea的CO2电解槽内的相变。该模型预测关键性能指标,包括CO法拉第效率、能量和质量转换效率、电极泛洪和盐沉淀。模拟结果确定了最佳操作策略:阴极侧在10°C下冷却,在8°atm下升高压力,阳极侧在80°C下加热,与基线条件相比,总能源效率提高了42.4%。这些发现强调了精确的热和质量传输管理在推进可扩展的CO2电解槽技术中的重要性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
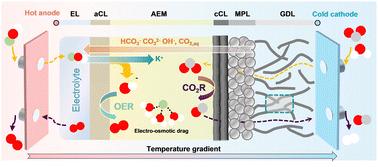
Mass and thermal management strategies for MEA-based CO2 electrolyzers enabled by physics-based modeling
Membrane electrode assembly (MEA)-based CO2 electrolyzers are promising for electrochemical CO2 reduction (CO2R) due to their compact design and high current densities. However, performance and durability are often limited by mass transport constraints, thermal gradients, and salt precipitation. We present a comprehensive, non-isothermal, physics-based model that captures multiphase transport of gaseous, liquid, and ionic species, coupled with heat generation, electrochemical reactions, and phase transitions within an MEA-based CO2 electrolyzer. This model predicts key performance indicators, including CO faradaic efficiency, energy and mass conversion efficiencies, electrode flooding, and salt precipitation. Simulation results identify optimal operating strategies: cathode-side cooling at 10 °C, elevated pressure at 8 atm, and anode-side heating at 80 °C, collectively improving energy efficiency by 42.4% compared to baseline conditions. These findings underscore the importance of precise thermal and mass transport management in advancing scalable CO2 electrolyzer technologies.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Physical Chemistry Chemical Physics
化学-物理:原子、分子和化学物理
CiteScore
5.50
自引率
9.10%
发文量
2675
审稿时长
2.0 months
期刊介绍:
Physical Chemistry Chemical Physics (PCCP) is an international journal co-owned by 19 physical chemistry and physics societies from around the world. This journal publishes original, cutting-edge research in physical chemistry, chemical physics and biophysical chemistry. To be suitable for publication in PCCP, articles must include significant innovation and/or insight into physical chemistry; this is the most important criterion that reviewers and Editors will judge against when evaluating submissions.
The journal has a broad scope and welcomes contributions spanning experiment, theory, computation and data science. Topical coverage includes spectroscopy, dynamics, kinetics, statistical mechanics, thermodynamics, electrochemistry, catalysis, surface science, quantum mechanics, quantum computing and machine learning. Interdisciplinary research areas such as polymers and soft matter, materials, nanoscience, energy, surfaces/interfaces, and biophysical chemistry are welcomed if they demonstrate significant innovation and/or insight into physical chemistry. Joined experimental/theoretical studies are particularly appreciated when complementary and based on up-to-date approaches.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


