高风险肺栓塞的血栓切除术-装置与溶栓:鱼雷- nl研究者发起,学术资助,多中心,开放标签随机对照试验的原理和设计。
IF 3.4
3区 医学
Q1 HEMATOLOGY
引用次数: 0
摘要
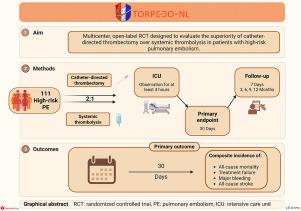
背景:在急性高危肺栓塞(PE)患者中,导管定向取栓(CDT)是一种有希望的替代全剂量溶栓的方法,有望对肺动脉凝块负担有更直接的影响,并且具有更好的安全性。目前尚无评估高危患者CDT安全性和有效性的随机试验。方法和结果:鱼雷- nl研究是一项由研究者发起、公共资助、多中心、开放标签的随机对照试验,旨在评估高危PE患者CDT治疗优于全身溶栓治疗的优越性。患有以下情况的成年人:1)确诊急性PE, 2)死亡率高,3)CDT可用且技术可行,将按2:1随机分为CDT和全身性溶栓治疗。主要结局是第30天全因死亡率、治疗失败、大出血和全因卒中的综合发生率。次要结局包括第7天的理想结局排名(DOOR)、住院时间、患者报告的结局包括生活质量和症状负担、功能恢复和1年成本效益。该试验预计将招募111名患者,由荷兰卫生保健研究所、荷兰卫生研究与发展组织、荷兰心脏基金会以及Penumbra Inc.和Inari Medical的无限制资助。Clinicaltrials: gov编号:NCT06833827。结论:TORPEDO-NL是第一个公共资助的随机试验,旨在研究CDT治疗对高危PE患者的影响。该试验有望在修订国际指南中关于高风险PE治疗的建议方面发挥重要作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Thrombectomy in high-risk pulmonary embolism – device versus thrombolysis: rationale and design of the TORPEDO-NL investigator-initiated, academically-sponsored, multicenter, open-label randomized controlled trial
Background
Catheter-directed thrombectomy (CDT) is a promising alternative to full dose thrombolysis in patients with acute high-risk pulmonary embolism (PE), expected to have a more direct effect on pulmonary artery clot burden and a better safety profile. Randomized trials evaluating the safety and efficacy of CDT in high-risk patients are currently unavailable.
Methods and results
The TORPEDO-NL study is an investigator-initiated, publicly-funded, multicenter, open-label randomized controlled trial designed to evaluate the superiority of CDT over systemic thrombolysis in patients with high-risk PE. Adults with: 1) confirmed acute PE, 2) a high risk for mortality, and 3) CDT available and technically feasible, will be randomized 2:1 to CDT versus systemic thrombolysis. The primary outcome is the composite incidence of all-cause mortality, treatment failure, major bleeding, and all-cause stroke at day 30. Secondary outcomes include desirability of outcome ranking (DOOR) at day 7, length of hospital stay, patient-reported outcomes including quality of life and symptom burden, functional recovery, and 1-year cost-effectiveness. The trial anticipates recruiting 111 patients and is funded by the The Netherlands Health Care Institute, The Netherlands Organization for Health Research and Development, the Dutch Heart Foundation, and unrestricted grants from Penumbra Inc. and Inari Medical. ClinicalTrials.gov number, NCT06833827.
Conclusions
TORPEDO-NL is the first publicly-funded randomized trial to investigate the effect of CDT treatment specifically in high-risk PE patients. The trial is anticipated to play an important role in revising recommendations for high-risk PE treatment in international guidelines.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Thrombosis research
医学-外周血管病
CiteScore
14.60
自引率
4.00%
发文量
364
审稿时长
31 days
期刊介绍:
Thrombosis Research is an international journal dedicated to the swift dissemination of new information on thrombosis, hemostasis, and vascular biology, aimed at advancing both science and clinical care. The journal publishes peer-reviewed original research, reviews, editorials, opinions, and critiques, covering both basic and clinical studies. Priority is given to research that promises novel approaches in the diagnosis, therapy, prognosis, and prevention of thrombotic and hemorrhagic diseases.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


