天然产品激发的强效抗癌同系物:时间进展,构效关系(SAR)研究和未来展望。
IF 3
3区 医学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
癌症是全球死亡和发病的主要原因之一。天然产物在为临床治疗各种类型的癌症带来新疗法方面发挥了重要作用。然而,一些天然产物效力低,化学不稳定,药代动力学差,毒性高。因此,一种以天然产物为灵感的策略已经被用来克服天然产物在癌症药物发现中的局限性。在这里,我们提出了一个关键的审查,以描述如何药物化学家设计抗癌剂通过天然产品的启发策略。我们还说明了如何使用这种方法来克服癌症的耐药性。以天然产物为灵感的类似物/衍生物被分为不同的类别。我们的重点扩展到审查设计策略,体外/体内结果,以及结构-活性关系(SAR)研究的天然产品启发分子作为抗癌剂。我们希望这一综述能够启发更有效、更多样化的抗癌药物的开发。本文章由计算机程序翻译,如有差异,请以英文原文为准。
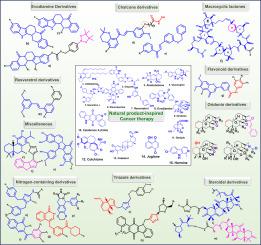
Natural-product-inspired potent anticancer congeners: chronological advancements, structure-activity relationship (SAR) studies and future perspectives
Cancer is among the leading causes of mortality and morbidity globally. Natural products have played a significant role in bringing novel therapies to the clinic for the treatment of various types of cancer. However, several natural products have low potency, chemical instability, poor pharmacokinetics, and high toxicity. Therefore, a natural-product-inspired strategy has been utilized to overcome the limitations of natural products in cancer drug discovery. Herein, we present a critical review to describe how medicinal chemists designed anti-cancer agents via a natural-product-inspired strategy. We have also illustrated how this approach is used to overcome drug resistance in cancer. The natural product-inspired analogues/derivatives were classified into different categories. Our focus extends to reviewing the design strategies, in vitro/in vivo results, and structure-activity relationship (SAR) studies of natural-product-inspired molecules as anti-cancer agents. We expect that this review will inspire the development of more effective and diversified anti-cancer agents.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioorganic & Medicinal Chemistry
医学-生化与分子生物学
CiteScore
6.80
自引率
2.90%
发文量
413
审稿时长
17 days
期刊介绍:
Bioorganic & Medicinal Chemistry provides an international forum for the publication of full original research papers and critical reviews on molecular interactions in key biological targets such as receptors, channels, enzymes, nucleotides, lipids and saccharides.
The aim of the journal is to promote a better understanding at the molecular level of life processes, and living organisms, as well as the interaction of these with chemical agents. A special feature will be that colour illustrations will be reproduced at no charge to the author, provided that the Editor agrees that colour is essential to the information content of the illustration in question.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


