Mn(II)、Co(II)和Zn(II)配合物与3-羟基黄酮的合成、光谱和光物理研究:对前列腺癌细胞抗癌活性的见解。
IF 3.2
2区 化学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
本文报道了Mn(II)、Co(II)和Zn(II)与3-羟基黄酮配合物的合成、光谱和光物理表征以及抗癌评价。配合物以1:2的金属与配体的摩尔比得到,生成固体单核物质,通式为ML2·nH2O,其中M = Mn(II), Co(II)或Zn(II), L - 3-羟黄酮。通过元素分析、热重分析(TG/DTG-DSC)、差示扫描量热法、紫外可见光谱、红外光谱、核磁共振、109AgNP LDI MS(光纤激光产生的银-109纳米粒子用于激光解吸/电离质谱)和荧光光谱对化合物进行了表征。光谱研究表明,通过3-羟基和羰基进行配位,导致配体的结构和电子修饰。荧光性质受到金属配位的强烈影响,其中锌-3-羟基黄酮由于激发态质子转移的改变而表现出最明显的发射变化。复合物对前列腺癌(DU-145)、正常前列腺(RWPE-2)和正常成纤维细胞(BJ)的细胞毒活性进行了评估。zn -3-羟基黄酮的抑癌活性最高,IC50为4.08 μM。所有复合物都表现出选择性的细胞毒性,即使浓度超过其IC50值,正常细胞系的存活率也很高。恢复试验显示,暴露于该复合物后,DU-145细胞有永久性的细胞毒性作用或部分再生。细胞凋亡的关键标志物磷脂酰丝氨酸外化的实时检测表明,诱导细胞凋亡是Mn(II)和Zn(II)复合物对前列腺癌细胞最可能的作用机制。本文章由计算机程序翻译,如有差异,请以英文原文为准。
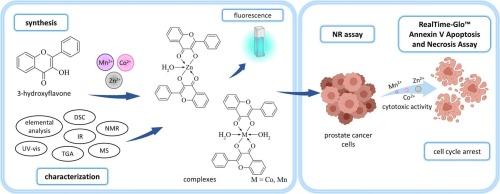
Synthesis, spectroscopic, and photophysical studies of Mn(II), Co(II), and Zn(II) complexes with 3-hydroxyflavone: Insights into anticancer activity against prostate cancer cells
This study reports the synthesis, spectroscopic and photophysical characterization, and anticancer evaluation of Mn(II), Co(II), and Zn(II) complexes with 3-hydroxyflavone. The complexes were obtained in a 1:2 metal-to-ligand molar ratio, yielding solid mononuclear species with the general formula ML2·nH2O, where M = Mn(II), Co(II), or Zn(II), and L⁻ = 3-hydroxyflavonate. The compounds were characterized by elemental analysis, thermogravimetric analysis (TG/DTG–DSC), differential scanning calorimetry, UV–Vis, FT-IR, NMR, 109AgNP LDI MS (Fiber Laser-Generated Silver-109 Nanoparticles for Laser Desorption/Ionization Mass Spectrometry), and fluorescence spectroscopy. Spectroscopic studies indicated coordination through 3-hydroxy and carbonyl groups, leading to structural and electronic modifications of the ligand. Fluorescence properties were strongly influenced by metal coordination, with Zn–3-hydroxyflavone showing the most pronounced emission changes due to altered excited-state intramolecular proton transfer. The cytotoxic activity of the complexes was evaluated against prostate cancer (DU-145), normal prostate (RWPE-2), and normal fibroblast (BJ) cell lines. Zn–3-hydroxyflavone exhibited the highest anticancer activity, with a half-maximal inhibitory concentration (IC50) of 4.08 μM. All complexes displayed selective cytotoxicity, as evidenced by the high viability of normal cell lines even at concentrations exceeding their IC50 values. Recovery assays revealed either a permanent cytotoxic effect or partial regeneration of DU-145 cells following exposure to the complexes. Real-time detection of phosphatidylserine externalization, a key marker of apoptosis, indicated that induction of apoptosis is the most probable mechanism of action for the Mn(II) and Zn(II) complexes against prostate cancer cells.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Inorganic Biochemistry
生物-生化与分子生物学
CiteScore
7.00
自引率
10.30%
发文量
336
审稿时长
41 days
期刊介绍:
The Journal of Inorganic Biochemistry is an established international forum for research in all aspects of Biological Inorganic Chemistry. Original papers of a high scientific level are published in the form of Articles (full length papers), Short Communications, Focused Reviews and Bioinorganic Methods. Topics include: the chemistry, structure and function of metalloenzymes; the interaction of inorganic ions and molecules with proteins and nucleic acids; the synthesis and properties of coordination complexes of biological interest including both structural and functional model systems; the function of metal- containing systems in the regulation of gene expression; the role of metals in medicine; the application of spectroscopic methods to determine the structure of metallobiomolecules; the preparation and characterization of metal-based biomaterials; and related systems. The emphasis of the Journal is on the structure and mechanism of action of metallobiomolecules.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


